Tuesday Poster Session
Category: IBD
P5370 - Safety of Live Vaccines in Patients With Inflammatory Bowel Disease on Biologic Therapy: A Propensity Score Matched Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- NW
Niven Wang, DO
University of New Mexico
Albuquerque, NM
Presenting Author(s)
Niven Wang, DO1, Ahmed Telbany, MD1, Abdelrahman Yousef, MD2, Daniel Paverini, MD1, Swathi Paleti, MD3, Christopher Chang, MD1
1University of New Mexico, Albuquerque, NM; 2University of New Mexico Hospital, Albuquerque, NM; 3University of New Mexico Health Sciences Center, Albuquerque, NM
Introduction: Live vaccines are generally avoided in patients with inflammatory bowel disease (IBD) on biologic therapy due to concerns about infection risk. However, real-world data evaluating the safety of live vaccines in this population are limited. This study aimed to evaluate adverse outcomes following live vaccination in IBD patients receiving biologic treatment.
Methods: We conducted a retrospective cohort study using a large, multi-institutional electronic health record database (TriNetX). Adults with IBD receiving biologic therapy were divided into two cohorts: those who received a live vaccine and those who did not. Biologic therapies included tumor necrosis factor inhibitors (infliximab, adalimumab), integrin antagonists (vedolizumab), interleukin-12/23 inhibitors (ustekinumab), interleukin-23 inhibitors (risankizumab, guselkumab), and Janus kinase inhibitors (tofacitinib, upadacitinib). Live vaccines included measles-mumps-rubella (MMR), Varivax (varicella vaccine), and yellow fever. Propensity score matching was performed based on age, sex, IBD subtype (Crohn’s disease vs ulcerative colitis), and immunologic agent class. Patients with the outcome prior to the risk window were excluded. Adverse outcomes were assessed within 12 months of vaccination and included inpatient visits, emergency department (ED) visits, fever, rash, pneumonia, sepsis, and encephalitis.
Results: A total of 758 patients were included in each matched cohort. No significant increase in adverse events was observed in the vaccinated group compared to the unvaccinated group. Inpatient visits occurred in 8.7% vs 9.1% (risk ratio [RR] 0.95; 95% confidence interval [CI]: 0.55–1.65; p=0.866), and ED visits in 6.4% vs 8.3% (RR 0.78; 95% CI: 0.48–1.25; p=0.298). There were no significant differences in fever (2.5% vs 2.9%), rash (4.1% vs 5.3%), pneumonia (1.4% vs 1.4%), sepsis (1.4% vs 1.4%), or encephalitis (0% in both).
Discussion: Live vaccines were not associated with an increased risk of adverse outcomes within 12 months in IBD patients receiving biologic therapy. These real-world findings support the short-term safety of live vaccines in selected immunosuppressed IBD patients and may inform vaccination guidelines. This study also provides a basis for future prospective randomized trials to more definitively assess vaccine safety in this population.
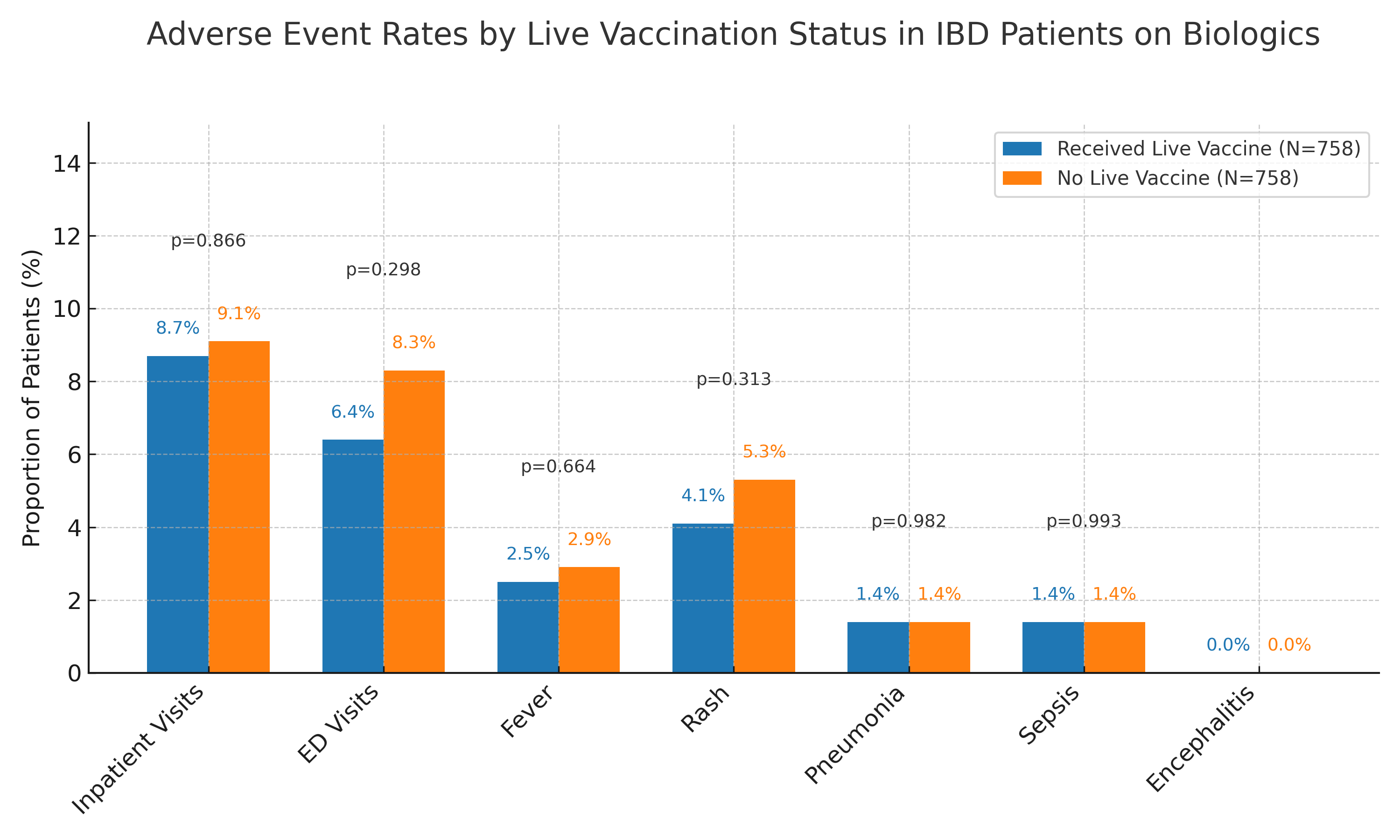
Figure: Adverse event rates within 12 months following live vaccination in patients with inflammatory bowel disease (IBD) on biologic therapy.
Disclosures:
Niven Wang indicated no relevant financial relationships.
Ahmed Telbany indicated no relevant financial relationships.
Abdelrahman Yousef indicated no relevant financial relationships.
Daniel Paverini indicated no relevant financial relationships.
Swathi Paleti indicated no relevant financial relationships.
Christopher Chang indicated no relevant financial relationships.
Niven Wang, DO1, Ahmed Telbany, MD1, Abdelrahman Yousef, MD2, Daniel Paverini, MD1, Swathi Paleti, MD3, Christopher Chang, MD1. P5370 - Safety of Live Vaccines in Patients With Inflammatory Bowel Disease on Biologic Therapy: A Propensity Score Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of New Mexico, Albuquerque, NM; 2University of New Mexico Hospital, Albuquerque, NM; 3University of New Mexico Health Sciences Center, Albuquerque, NM
Introduction: Live vaccines are generally avoided in patients with inflammatory bowel disease (IBD) on biologic therapy due to concerns about infection risk. However, real-world data evaluating the safety of live vaccines in this population are limited. This study aimed to evaluate adverse outcomes following live vaccination in IBD patients receiving biologic treatment.
Methods: We conducted a retrospective cohort study using a large, multi-institutional electronic health record database (TriNetX). Adults with IBD receiving biologic therapy were divided into two cohorts: those who received a live vaccine and those who did not. Biologic therapies included tumor necrosis factor inhibitors (infliximab, adalimumab), integrin antagonists (vedolizumab), interleukin-12/23 inhibitors (ustekinumab), interleukin-23 inhibitors (risankizumab, guselkumab), and Janus kinase inhibitors (tofacitinib, upadacitinib). Live vaccines included measles-mumps-rubella (MMR), Varivax (varicella vaccine), and yellow fever. Propensity score matching was performed based on age, sex, IBD subtype (Crohn’s disease vs ulcerative colitis), and immunologic agent class. Patients with the outcome prior to the risk window were excluded. Adverse outcomes were assessed within 12 months of vaccination and included inpatient visits, emergency department (ED) visits, fever, rash, pneumonia, sepsis, and encephalitis.
Results: A total of 758 patients were included in each matched cohort. No significant increase in adverse events was observed in the vaccinated group compared to the unvaccinated group. Inpatient visits occurred in 8.7% vs 9.1% (risk ratio [RR] 0.95; 95% confidence interval [CI]: 0.55–1.65; p=0.866), and ED visits in 6.4% vs 8.3% (RR 0.78; 95% CI: 0.48–1.25; p=0.298). There were no significant differences in fever (2.5% vs 2.9%), rash (4.1% vs 5.3%), pneumonia (1.4% vs 1.4%), sepsis (1.4% vs 1.4%), or encephalitis (0% in both).
Discussion: Live vaccines were not associated with an increased risk of adverse outcomes within 12 months in IBD patients receiving biologic therapy. These real-world findings support the short-term safety of live vaccines in selected immunosuppressed IBD patients and may inform vaccination guidelines. This study also provides a basis for future prospective randomized trials to more definitively assess vaccine safety in this population.

Figure: Adverse event rates within 12 months following live vaccination in patients with inflammatory bowel disease (IBD) on biologic therapy.
Disclosures:
Niven Wang indicated no relevant financial relationships.
Ahmed Telbany indicated no relevant financial relationships.
Abdelrahman Yousef indicated no relevant financial relationships.
Daniel Paverini indicated no relevant financial relationships.
Swathi Paleti indicated no relevant financial relationships.
Christopher Chang indicated no relevant financial relationships.
Niven Wang, DO1, Ahmed Telbany, MD1, Abdelrahman Yousef, MD2, Daniel Paverini, MD1, Swathi Paleti, MD3, Christopher Chang, MD1. P5370 - Safety of Live Vaccines in Patients With Inflammatory Bowel Disease on Biologic Therapy: A Propensity Score Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
