Tuesday Poster Session
Category: IBD
P5354 - Long-Term Outcomes of Biologic Therapy in IBD: A Meta-Analysis of Real-World and Randomized Data
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Prince Shah-Riar, MD (he/him/his)
DHR Health, Edinburg, Tx
Edinburg, TX
Presenting Author(s)
Prince Shah-Riar, MD1, Nowrin Tamanna, MBBS2, Umme Kulsum, MBBS3, Rishika Trivedi, MD4, Asif Zamir, MD, FACG5
1DHR Health, Edinburg, Tx, McAllen, TX; 2Chittagong Medical College,Bangladesh, Santa Clara, CA; 3Rangpur Medical College, Rangpur, Rangpur, Bangladesh; 4DHR Health, McAllen, TX; 5DHR Health Gastroenterology, Edinburg, TX
Introduction: Inflammatory Bowel Disease (IBD), including Crohn’s disease and ulcerative colitis, often necessitates long-term therapy to achieve and maintain remission. While biologics have transformed disease control, their long-term comparative effectiveness and safety across drug classes remain insufficiently synthesized. Most existing data are from controlled trials with limited external validity.
This study aimed to evaluate long-term clinical outcomes of biologic therapies in IBD using real-world and randomized controlled data, focusing on remission, mucosal healing, steroid independence, and adverse event profiles.
Methods: We conducted a PRISMA-compliant meta-analysis of randomized controlled trials (RCTs) and real-world cohort studies from 2020–2022. Databases searched included PubMed (n=796), Cochrane (n=403), ClinicalTrials.gov (n=361), and Google Scholar (n=64). Inclusion criteria were: (1) adult IBD patients treated with biologics, (2) ≥12 months follow-up, (3) sample size ≥50. Outcomes included clinical remission, mucosal healing, steroid-free remission, and serious adverse events (SAEs). Of 1,620 studies identified, 23 were eligible for quantitative synthesis. Data were pooled using a random-effects model. Risk of bias was assessed via Cochrane ROB-2.
Results: Among 7,842 patients across 23 studies:
Discussion: This meta-analysis confirms sustained benefits of biologic therapy in long-term IBD management, including robust remission and mucosal healing outcomes. Risk of adverse events remains acceptable, supporting continued use beyond induction. However, heterogeneity in definitions of remission and underreporting of steroid dependence in some studies highlight the need for standardized endpoints in future trials. Biologics are effective and well-tolerated for long-term IBD control, with clinically meaningful gains in remission and mucosal integrity. These findings advocate for early, sustained biologic use, especially in high-risk phenotypes, and call for head-to-head comparative trials with harmonized outcome reporting.
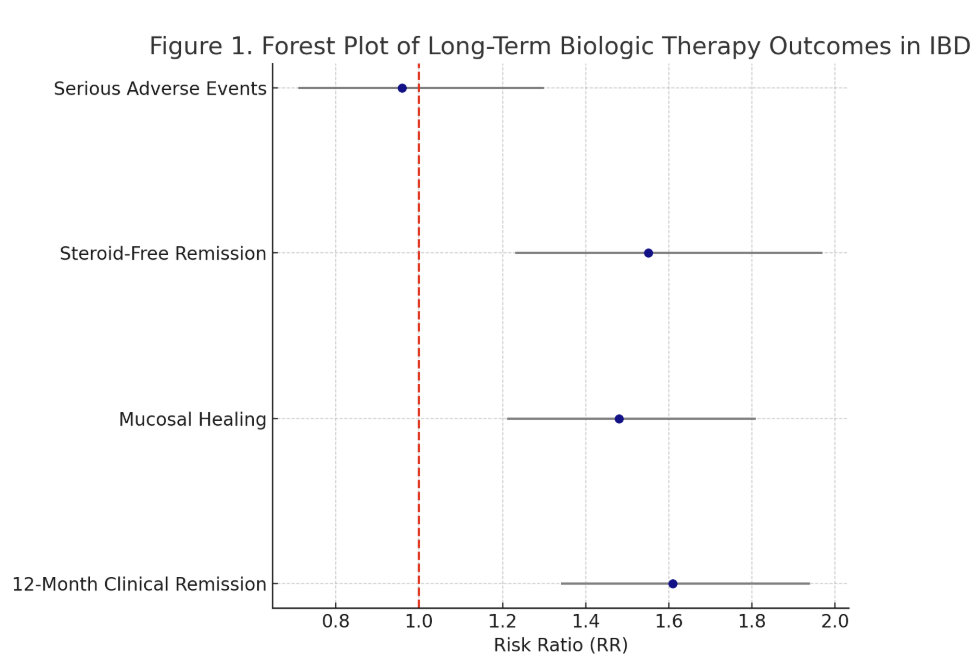
Figure: Figure 1 – Forest Plot of Biologic Outcomes
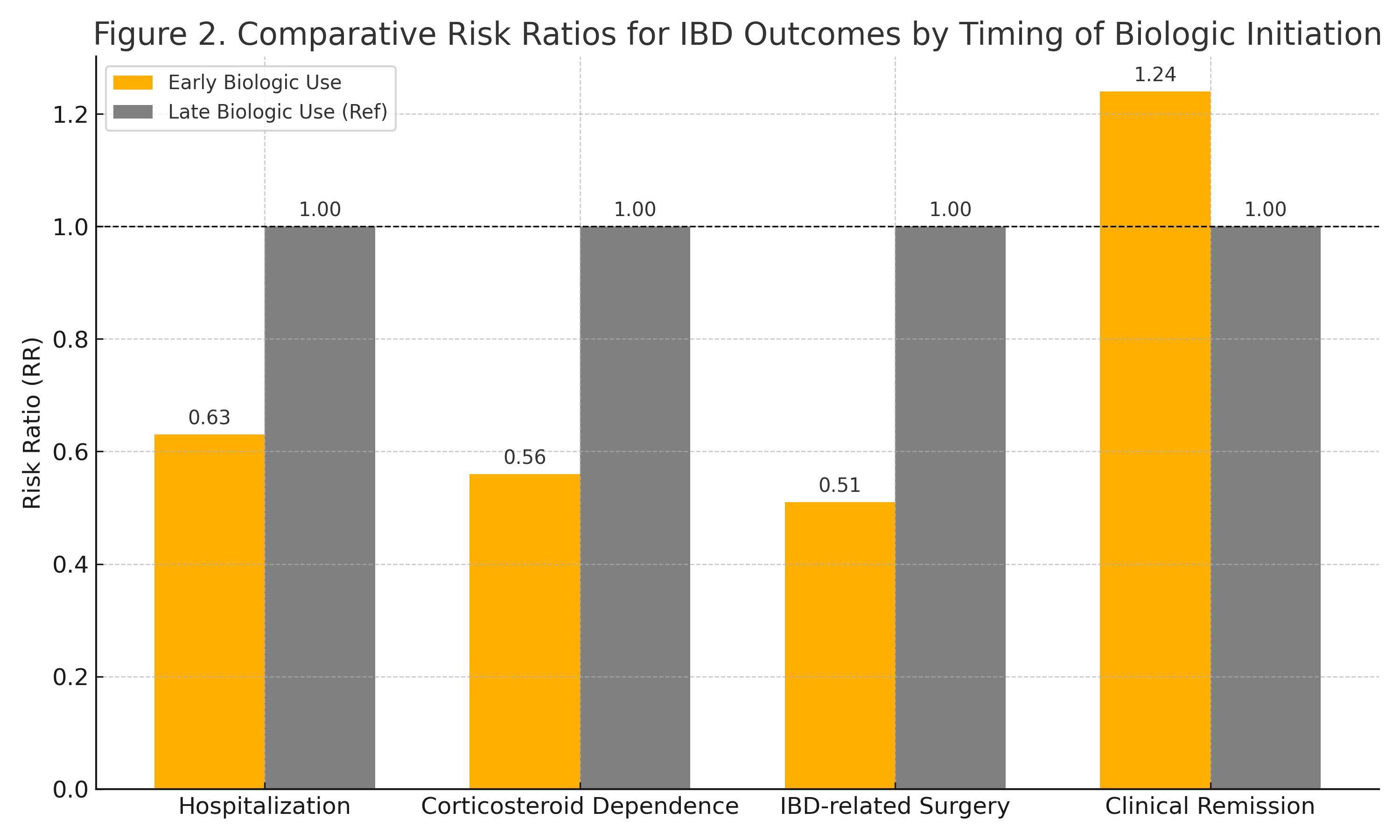
Figure: Figure 2: Comparative Risk Ratios for IBD Outcomes by Timing of Biologic Initiation
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Nowrin Tamanna indicated no relevant financial relationships.
Umme Kulsum indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Nowrin Tamanna, MBBS2, Umme Kulsum, MBBS3, Rishika Trivedi, MD4, Asif Zamir, MD, FACG5. P5354 - Long-Term Outcomes of Biologic Therapy in IBD: A Meta-Analysis of Real-World and Randomized Data, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1DHR Health, Edinburg, Tx, McAllen, TX; 2Chittagong Medical College,Bangladesh, Santa Clara, CA; 3Rangpur Medical College, Rangpur, Rangpur, Bangladesh; 4DHR Health, McAllen, TX; 5DHR Health Gastroenterology, Edinburg, TX
Introduction: Inflammatory Bowel Disease (IBD), including Crohn’s disease and ulcerative colitis, often necessitates long-term therapy to achieve and maintain remission. While biologics have transformed disease control, their long-term comparative effectiveness and safety across drug classes remain insufficiently synthesized. Most existing data are from controlled trials with limited external validity.
This study aimed to evaluate long-term clinical outcomes of biologic therapies in IBD using real-world and randomized controlled data, focusing on remission, mucosal healing, steroid independence, and adverse event profiles.
Methods: We conducted a PRISMA-compliant meta-analysis of randomized controlled trials (RCTs) and real-world cohort studies from 2020–2022. Databases searched included PubMed (n=796), Cochrane (n=403), ClinicalTrials.gov (n=361), and Google Scholar (n=64). Inclusion criteria were: (1) adult IBD patients treated with biologics, (2) ≥12 months follow-up, (3) sample size ≥50. Outcomes included clinical remission, mucosal healing, steroid-free remission, and serious adverse events (SAEs). Of 1,620 studies identified, 23 were eligible for quantitative synthesis. Data were pooled using a random-effects model. Risk of bias was assessed via Cochrane ROB-2.
Results: Among 7,842 patients across 23 studies:
- 12-month Clinical Remission: Achieved in 58% of patients (pooled RR 1.61; 95% CI: 1.34–1.94; I² = 29%)
- Mucosal Healing: Achieved in 46% (RR 1.48; 95% CI: 1.21–1.81; I² = 35%)
- Steroid-Free Remission: Documented in 42% (RR 1.55; 95% CI: 1.23–1.97; I² = 30%)
- Adverse Events: Serious infections occurred in 6.2%, treatment discontinuation due to AEs in 4.8%. No excess cancer risk observed during follow-up (RR 0.96; 95% CI: 0.71–1.30)
Discussion: This meta-analysis confirms sustained benefits of biologic therapy in long-term IBD management, including robust remission and mucosal healing outcomes. Risk of adverse events remains acceptable, supporting continued use beyond induction. However, heterogeneity in definitions of remission and underreporting of steroid dependence in some studies highlight the need for standardized endpoints in future trials. Biologics are effective and well-tolerated for long-term IBD control, with clinically meaningful gains in remission and mucosal integrity. These findings advocate for early, sustained biologic use, especially in high-risk phenotypes, and call for head-to-head comparative trials with harmonized outcome reporting.

Figure: Figure 1 – Forest Plot of Biologic Outcomes

Figure: Figure 2: Comparative Risk Ratios for IBD Outcomes by Timing of Biologic Initiation
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Nowrin Tamanna indicated no relevant financial relationships.
Umme Kulsum indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Nowrin Tamanna, MBBS2, Umme Kulsum, MBBS3, Rishika Trivedi, MD4, Asif Zamir, MD, FACG5. P5354 - Long-Term Outcomes of Biologic Therapy in IBD: A Meta-Analysis of Real-World and Randomized Data, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
