Tuesday Poster Session
Category: IBD
P5322 - Investigating Unmet Needs Among Advanced Therapy-Naïve Patients With Ulcerative Colitis Treated With Ustekinumab or Upadacitinib
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- CK
Caroline Kerner, MD, MSc (she/her/hers)
Johnson & Johnson
Horsham, PA
Presenting Author(s)
Freddy Caldera, DO, PhD, MS1, Maryia Zhdanava, MA2, Sumesh Kachroo, PhD3, Aditi Shah, MA4, Lilian Diaz, MSc2, Fengyi Jiang, MSc4, Caroline Kerner, MD, MSc3, Dominic Pilon, MA2, Jennifer Seminerio, MD5
1University of Wisconsin Hospitals and Clinics, Madison, WI; 2Analysis Group, Inc., Montreal, PQ, Canada; 3Johnson & Johnson, Horsham, PA; 4Analysis Group, Inc., Toronto, ON, Canada; 5Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL
Introduction: Ustekinumab, an interleukin (IL)-12/23 inhibitor, and upadacitinib, a Janus kinase inhibitor, are effective advanced therapies (ATs) for ulcerative colitis (UC), yet patients may experience suboptimal outcomes. This study sought to compare indicators of suboptimal treatment and healthcare resource utilization (HRU) between AT-naïve patients on ustekinumab or upadacitinib to identify unmet treatment needs.
Methods: This retrospective study used Komodo Research Data, a US medical and pharmacy claims database. Adults with UC who initiated ustekinumab or upadacitinib (index date) from 03/16/2022-11/30/2023 were included. Patients had ≥12 months of baseline insurance coverage without UC-indicated AT use pre-index. Baseline characteristics were balanced with inverse probability of treatment weights. A composite outcome of suboptimal treatment included: UC-related acute care visit, UC-related surgery, AT switch, augmentation with corticosteroids (CS), immunomodulators or 5-aminosalicylic acid (5-ASA), or dose escalation. Time to event from maintenance phase was analyzed with weighted Kaplan-Meier and Cox proportional hazards models. Patients without event were censored at end of index therapy supply. Poisson regression was used to compare HRU between cohorts from index date.
Results: 1,167 patients initiated ustekinumab and 268 patients initiated upadacitinib; characteristics were balanced for ustekinumab vs upadacitinib (mean age: 45.8 vs 46.0 years; female: 47.3% vs 49.5%; CS use: 76.0% vs 76.9%; mean follow-up: 9.2 vs 9.1 months). After 6 months, risk of any suboptimal treatment was significantly higher in patients on ustekinumab vs upadacitinib (52.8% vs 37.9%; hazard ratio=1.48; p< 0.001), driven by immunomodulator or 5-ASA augmentation (34.5% vs 26.1%). There was no difference in risk of severe indicators of suboptimal treatment for ustekinumab vs upadacitinib, including surgery (0.4% vs 0.3%), acute care visits (6.8% vs 4.8%), AT switch (5.7% vs 4.2%), and CS augmentation (20.9% vs 14.4%; Figure 1). HRU was generally similar between cohorts post-index, with fewer emergency room visits among the ustekinumab cohort (Figure 2).
Discussion: Large proportions of AT-naïve patients with UC experience suboptimal treatment on ustekinumab or upadacitinib, with similar risks of severe indicators, consistent with similar HRU after treatment initiation. These findings highlight unmet treatment needs that may be addressed by recently approved IL-23 inhibitors.
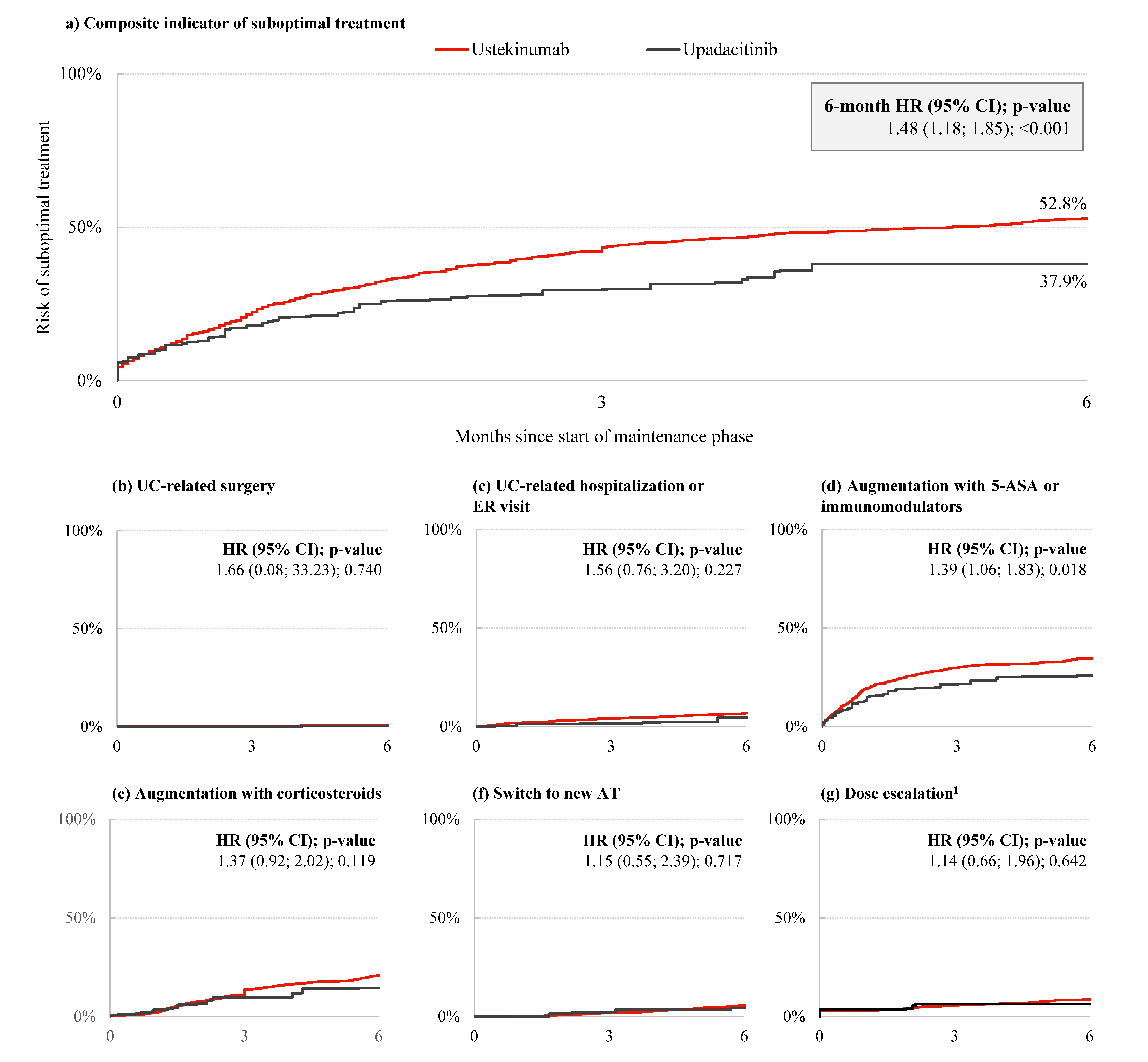
Figure: Figure 1. Kaplan-Meier risk of suboptimal treatment during follow-up period.
Abbreviations: ASA: aminosalicylic acid; AT: advanced therapy; ER: emergency room; HR: hazard ratio; UC: ulcerative colitis.
Note: 1. Defined as 100% (ustekinumab) or 50% (upadacitinib) increase relative to the labeled maintenance dose.
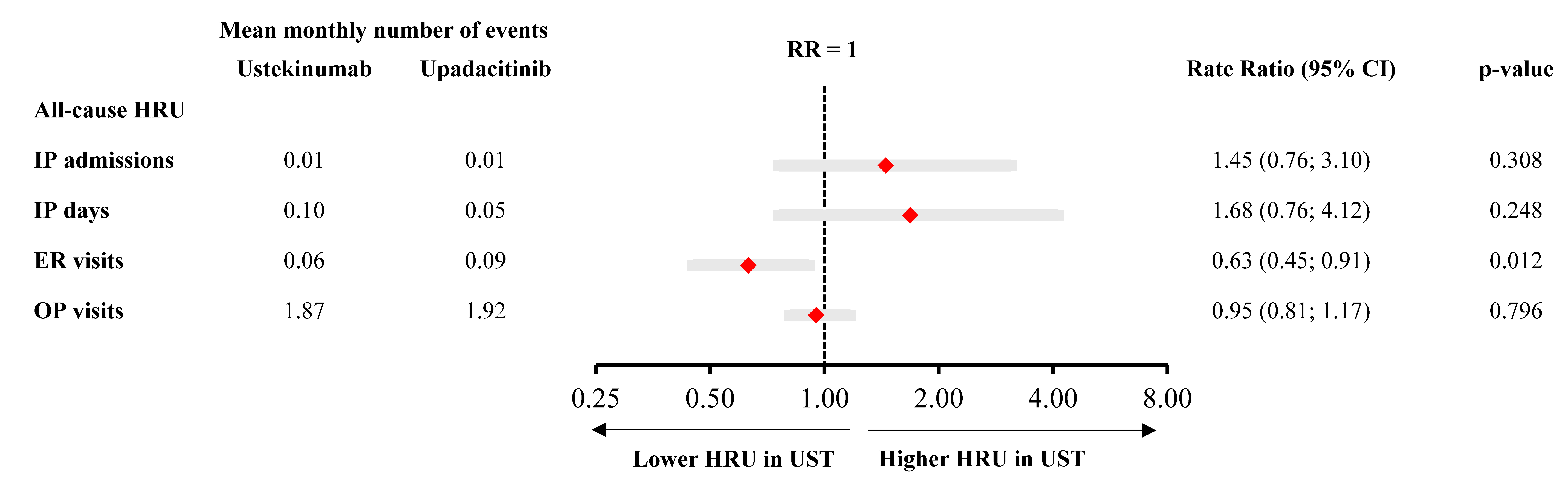
Figure: Figure 2: HRU during follow-up period.
Abbreviations: CI: confidence interval; HRU: healthcare resource utilization; IP: inpatient; ER: emergency room; OP: outpatient; RR: rate ratio; UST: ustekinumab.
Disclosures:
Freddy Caldera: GSK – Consultant. GSK – Grant/Research Support. Janssen – Consultant. Novavax – Grant/Research Support.
Maryia Zhdanava: Analysis Group, Inc. – Employee. Johnson & Johnson – I am an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson.
Sumesh Kachroo: Johnson & Johnson – Employee, Stock Options.
Aditi Shah: Analysis Group, Inc. – Employee. Johnson & Johnson – I am an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson.
Lilian Diaz: Analysis Group, Inc. – Employee. Johnson & Johnson – I am an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson.
Fengyi Jiang: Analysis Group, Inc. – Employee. Johnson & Johnson – I am an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson.
Caroline Kerner: Johnson & Johnson – Employee, Stock Options.
Dominic Pilon: Analysis Group, Inc. – Employee. Johnson & Johnson – I am an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson.
Jennifer Seminerio: Abbvie – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant.
Freddy Caldera, DO, PhD, MS1, Maryia Zhdanava, MA2, Sumesh Kachroo, PhD3, Aditi Shah, MA4, Lilian Diaz, MSc2, Fengyi Jiang, MSc4, Caroline Kerner, MD, MSc3, Dominic Pilon, MA2, Jennifer Seminerio, MD5. P5322 - Investigating Unmet Needs Among Advanced Therapy-Naïve Patients With Ulcerative Colitis Treated With Ustekinumab or Upadacitinib, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Wisconsin Hospitals and Clinics, Madison, WI; 2Analysis Group, Inc., Montreal, PQ, Canada; 3Johnson & Johnson, Horsham, PA; 4Analysis Group, Inc., Toronto, ON, Canada; 5Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL
Introduction: Ustekinumab, an interleukin (IL)-12/23 inhibitor, and upadacitinib, a Janus kinase inhibitor, are effective advanced therapies (ATs) for ulcerative colitis (UC), yet patients may experience suboptimal outcomes. This study sought to compare indicators of suboptimal treatment and healthcare resource utilization (HRU) between AT-naïve patients on ustekinumab or upadacitinib to identify unmet treatment needs.
Methods: This retrospective study used Komodo Research Data, a US medical and pharmacy claims database. Adults with UC who initiated ustekinumab or upadacitinib (index date) from 03/16/2022-11/30/2023 were included. Patients had ≥12 months of baseline insurance coverage without UC-indicated AT use pre-index. Baseline characteristics were balanced with inverse probability of treatment weights. A composite outcome of suboptimal treatment included: UC-related acute care visit, UC-related surgery, AT switch, augmentation with corticosteroids (CS), immunomodulators or 5-aminosalicylic acid (5-ASA), or dose escalation. Time to event from maintenance phase was analyzed with weighted Kaplan-Meier and Cox proportional hazards models. Patients without event were censored at end of index therapy supply. Poisson regression was used to compare HRU between cohorts from index date.
Results: 1,167 patients initiated ustekinumab and 268 patients initiated upadacitinib; characteristics were balanced for ustekinumab vs upadacitinib (mean age: 45.8 vs 46.0 years; female: 47.3% vs 49.5%; CS use: 76.0% vs 76.9%; mean follow-up: 9.2 vs 9.1 months). After 6 months, risk of any suboptimal treatment was significantly higher in patients on ustekinumab vs upadacitinib (52.8% vs 37.9%; hazard ratio=1.48; p< 0.001), driven by immunomodulator or 5-ASA augmentation (34.5% vs 26.1%). There was no difference in risk of severe indicators of suboptimal treatment for ustekinumab vs upadacitinib, including surgery (0.4% vs 0.3%), acute care visits (6.8% vs 4.8%), AT switch (5.7% vs 4.2%), and CS augmentation (20.9% vs 14.4%; Figure 1). HRU was generally similar between cohorts post-index, with fewer emergency room visits among the ustekinumab cohort (Figure 2).
Discussion: Large proportions of AT-naïve patients with UC experience suboptimal treatment on ustekinumab or upadacitinib, with similar risks of severe indicators, consistent with similar HRU after treatment initiation. These findings highlight unmet treatment needs that may be addressed by recently approved IL-23 inhibitors.

Figure: Figure 1. Kaplan-Meier risk of suboptimal treatment during follow-up period.
Abbreviations: ASA: aminosalicylic acid; AT: advanced therapy; ER: emergency room; HR: hazard ratio; UC: ulcerative colitis.
Note: 1. Defined as 100% (ustekinumab) or 50% (upadacitinib) increase relative to the labeled maintenance dose.

Figure: Figure 2: HRU during follow-up period.
Abbreviations: CI: confidence interval; HRU: healthcare resource utilization; IP: inpatient; ER: emergency room; OP: outpatient; RR: rate ratio; UST: ustekinumab.
Disclosures:
Freddy Caldera: GSK – Consultant. GSK – Grant/Research Support. Janssen – Consultant. Novavax – Grant/Research Support.
Maryia Zhdanava: Analysis Group, Inc. – Employee. Johnson & Johnson – I am an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson.
Sumesh Kachroo: Johnson & Johnson – Employee, Stock Options.
Aditi Shah: Analysis Group, Inc. – Employee. Johnson & Johnson – I am an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson.
Lilian Diaz: Analysis Group, Inc. – Employee. Johnson & Johnson – I am an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson.
Fengyi Jiang: Analysis Group, Inc. – Employee. Johnson & Johnson – I am an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson.
Caroline Kerner: Johnson & Johnson – Employee, Stock Options.
Dominic Pilon: Analysis Group, Inc. – Employee. Johnson & Johnson – I am an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson.
Jennifer Seminerio: Abbvie – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant.
Freddy Caldera, DO, PhD, MS1, Maryia Zhdanava, MA2, Sumesh Kachroo, PhD3, Aditi Shah, MA4, Lilian Diaz, MSc2, Fengyi Jiang, MSc4, Caroline Kerner, MD, MSc3, Dominic Pilon, MA2, Jennifer Seminerio, MD5. P5322 - Investigating Unmet Needs Among Advanced Therapy-Naïve Patients With Ulcerative Colitis Treated With Ustekinumab or Upadacitinib, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
