Tuesday Poster Session
Category: IBD
P5315 - Major Adverse Cardiovascular Events in Ulcerative Colitis Patients Treated with Vedolizumab, Tofacitinib, or Mesalamine
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Julton Tomanguillo Chumbe, MD
Charleston Area Medical Center
Charleston, WV
Presenting Author(s)
Julton Tomanguillo, MD, Nisar Amin, MD, Ebubekir Daglilar, MD, Harleen Chela, MD
Charleston Area Medical Center, Charleston, WV
Introduction: While Vedolizumab, Tofacitinib, and Mesalamine can be effective in managing ulcerative colitis (UC), concerns have been raised regarding their potential cardiovascular risks. This study aims to evaluate rate of MACE among UC patients receiving Vedolizumab, Tofacitinib, or Mesalamine.
Methods: We queried TrinetX – a Research Network - of 94 healthcare organizations between 2008 and 2025. UC patients were then divided into three cohorts based on their respective treatments: vedolizumab, tofacitinib, and mesalamine. The cohorts treated with vedolizumab and mesalamine were each compared to the tofacitinib cohort. A propensity score matching (PSM) of a 1:1 was performed to match covariates (age at index, sex, race, CAD, tobacco use, CKD, COPD, HTN, hyperlipidemia, body mass index). To account for multiple cohort comparisons and mitigate Type I errors, Bonferroni correction was applied, with a significant P value set at < 0.017. We compared rates of major adverse cardiovascular events (MACE), and rate of myocardial infarction (MI), stroke or transitory ischemic attack (CVA), death, heart failure (HF), revascularization (CABG and percutaneous coronary intervention), and atrial fibrillation or flutter, at 1, 3, 5 years intervals. .
Results: Total of 101,494 UC patients that either received Vedolizumab, Mesalamine, or Tofacitinib were included in the analysis. Of these, 8.98% (n=9,123%) received Vedolizumab, 1.99% (n=2,025) Tofacitinib, and 89.03% (n=90,343) mesalamine (Table 1 and 2). Subsequently, well-matched cohorts were created using a 1:1 PSM between Vedolizumab and Tofacitinib cohorts (2,247/2,247), and Tofacitinib and Mesalamine Cohorts (2,249/2,249). Vedolizumab and Tofacitinib cohorts were compared; no significant difference was found rate in the rate of MACE (0.006% vs 0.007%, p=0.57), MI (0.004% vs 0.004%, p=1.00), CVA (0.004% vs 0.004%, p=1.00), death (0.004% vs 0.005%, p=1.00), HF (0.004% vs 0.004%, p=1.00), revascularization (0.004% vs 0%, p=N/A), and atrial fibrillation or flutter (0.004% vs 0.004%, p=0.82) at 1-year. Similar findings were observed at 3 and 5 years. Additionally, when Tofacitinib and Mesalamine cohorts were compared, similar findings were observed at 1, 3 and 5 year intervals.
Discussion: This study shows that tofacitinib is not associated with higher risk for MACE as compared to other therapies used in UC. Given concerns that were raised in patients with rheumatoid arthritis and use of tofacitinib, this study does not reveal such concerns in UC.
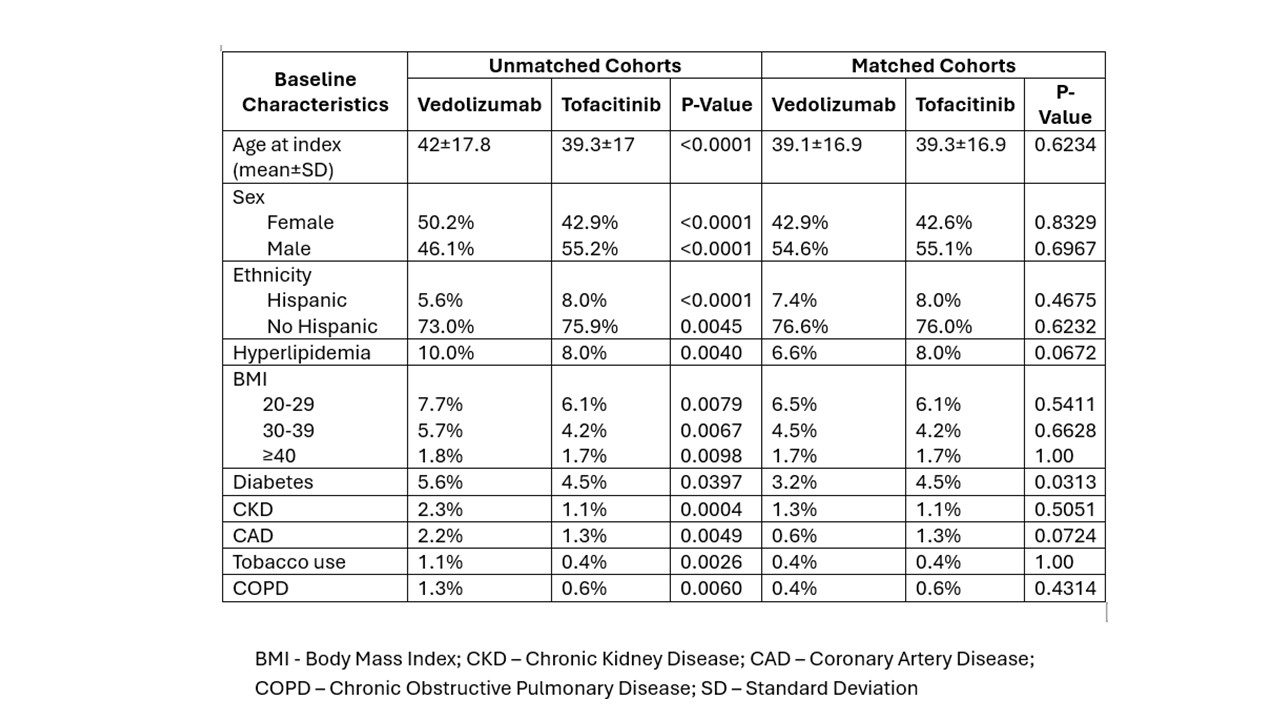
Figure: Table1: Baseline Characteristics between Patient receiving Vedolizumab and Tofacitinib
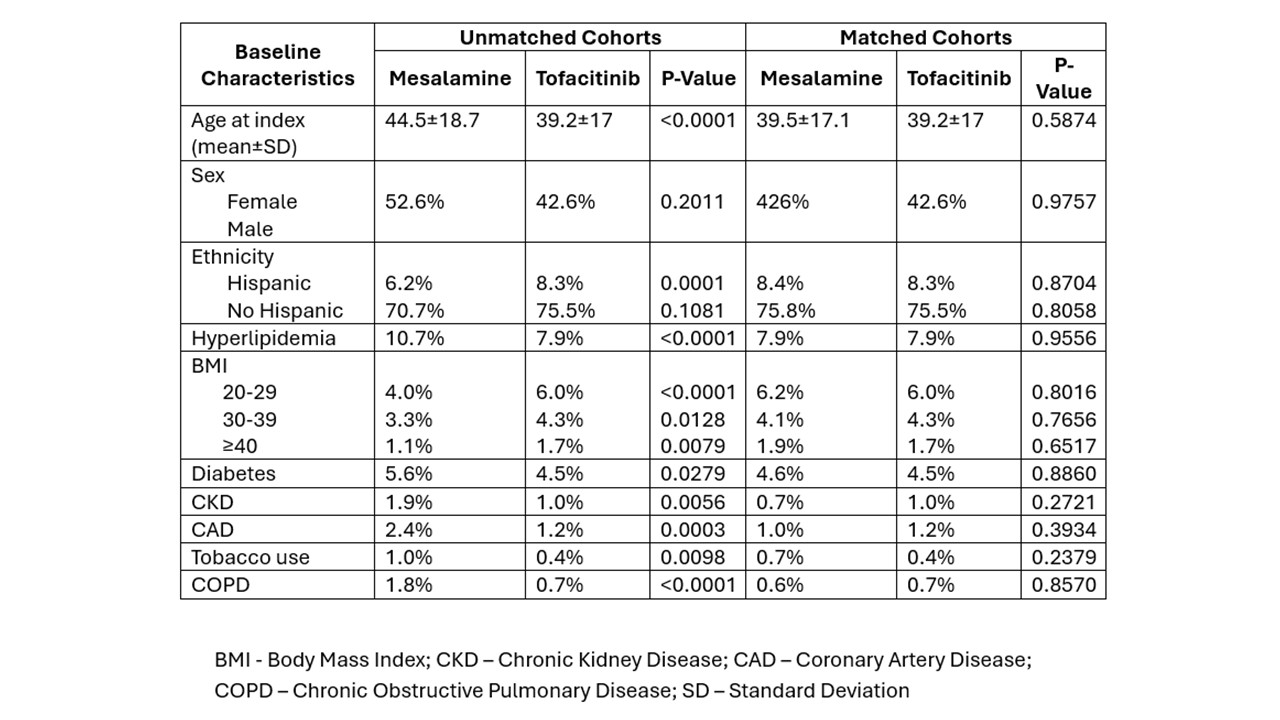
Figure: Table2: Baseline Characteristics between Patient receiving Mesalamine and Tofacitinib
Disclosures:
Julton Tomanguillo indicated no relevant financial relationships.
Nisar Amin indicated no relevant financial relationships.
Ebubekir Daglilar indicated no relevant financial relationships.
Harleen Chela indicated no relevant financial relationships.
Julton Tomanguillo, MD, Nisar Amin, MD, Ebubekir Daglilar, MD, Harleen Chela, MD. P5315 - Major Adverse Cardiovascular Events in Ulcerative Colitis Patients Treated with Vedolizumab, Tofacitinib, or Mesalamine, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Charleston Area Medical Center, Charleston, WV
Introduction: While Vedolizumab, Tofacitinib, and Mesalamine can be effective in managing ulcerative colitis (UC), concerns have been raised regarding their potential cardiovascular risks. This study aims to evaluate rate of MACE among UC patients receiving Vedolizumab, Tofacitinib, or Mesalamine.
Methods: We queried TrinetX – a Research Network - of 94 healthcare organizations between 2008 and 2025. UC patients were then divided into three cohorts based on their respective treatments: vedolizumab, tofacitinib, and mesalamine. The cohorts treated with vedolizumab and mesalamine were each compared to the tofacitinib cohort. A propensity score matching (PSM) of a 1:1 was performed to match covariates (age at index, sex, race, CAD, tobacco use, CKD, COPD, HTN, hyperlipidemia, body mass index). To account for multiple cohort comparisons and mitigate Type I errors, Bonferroni correction was applied, with a significant P value set at < 0.017. We compared rates of major adverse cardiovascular events (MACE), and rate of myocardial infarction (MI), stroke or transitory ischemic attack (CVA), death, heart failure (HF), revascularization (CABG and percutaneous coronary intervention), and atrial fibrillation or flutter, at 1, 3, 5 years intervals. .
Results: Total of 101,494 UC patients that either received Vedolizumab, Mesalamine, or Tofacitinib were included in the analysis. Of these, 8.98% (n=9,123%) received Vedolizumab, 1.99% (n=2,025) Tofacitinib, and 89.03% (n=90,343) mesalamine (Table 1 and 2). Subsequently, well-matched cohorts were created using a 1:1 PSM between Vedolizumab and Tofacitinib cohorts (2,247/2,247), and Tofacitinib and Mesalamine Cohorts (2,249/2,249). Vedolizumab and Tofacitinib cohorts were compared; no significant difference was found rate in the rate of MACE (0.006% vs 0.007%, p=0.57), MI (0.004% vs 0.004%, p=1.00), CVA (0.004% vs 0.004%, p=1.00), death (0.004% vs 0.005%, p=1.00), HF (0.004% vs 0.004%, p=1.00), revascularization (0.004% vs 0%, p=N/A), and atrial fibrillation or flutter (0.004% vs 0.004%, p=0.82) at 1-year. Similar findings were observed at 3 and 5 years. Additionally, when Tofacitinib and Mesalamine cohorts were compared, similar findings were observed at 1, 3 and 5 year intervals.
Discussion: This study shows that tofacitinib is not associated with higher risk for MACE as compared to other therapies used in UC. Given concerns that were raised in patients with rheumatoid arthritis and use of tofacitinib, this study does not reveal such concerns in UC.

Figure: Table1: Baseline Characteristics between Patient receiving Vedolizumab and Tofacitinib

Figure: Table2: Baseline Characteristics between Patient receiving Mesalamine and Tofacitinib
Disclosures:
Julton Tomanguillo indicated no relevant financial relationships.
Nisar Amin indicated no relevant financial relationships.
Ebubekir Daglilar indicated no relevant financial relationships.
Harleen Chela indicated no relevant financial relationships.
Julton Tomanguillo, MD, Nisar Amin, MD, Ebubekir Daglilar, MD, Harleen Chela, MD. P5315 - Major Adverse Cardiovascular Events in Ulcerative Colitis Patients Treated with Vedolizumab, Tofacitinib, or Mesalamine, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
