Tuesday Poster Session
Category: IBD
P5313 - Real-World Utility of Vedolizumab Clinical Decision Support Tool for Crohn’s Disease
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Anish Kuriakose Kuzhiyanjal, MBBS, MD, MRCP
Northern Care Alliance NHS Foundation Trust
Manchester, England, United Kingdom
Presenting Author(s)
Award: ACG Outstanding Research Award in the IBD Category (Trainee)
Award: ACG Presidential Poster Award
Anish Kuriakose Kuzhiyanjal, MBBS, MD, MRCP1, Sarah Rhodes, MSc, PhD2, Bernadett Farkas, MD3, Irene Latras-Cortés, MBBS, MD, MS4, Emily Granger, MBChB5, Stefanos Kateroglou, MD, MRCP1, Nzubechukwu Samuel. Ozokwelu, MBBS, MRCP6, Usman Saleem Raza, MBBS, MRCP7, Emma Nixon, MRCP8, Livia Lontai, MD9, Orsolya Menyhárt, MD10, Tibor Gyökeres, MD11, Mostafa I. Afifi, MBBCH, BSc012, Klaudia Farkas, MD, PhD3, Tamás Molnár, MD, PhD3, Parambir S. Dulai, MD13, Jimmy Limdi, MD14
1Northern Care Alliance NHS Foundation Trust, Manchester, England, United Kingdom; 2University of Manchester, Manchester, England, United Kingdom; 3University of Szeged, Szeged, Csongrad, Hungary; 4León University Hospital, León, Castilla y Leon, Spain; 5East Lancashire Hospitals NHS Trust, Blackburn, England, United Kingdom; 6Northern Care Alliance, Greater Manchester, England, United Kingdom; 7Salford Royal Hospital, Salford, England, United Kingdom; 8Northern Care alliance, Salford, England, United Kingdom; 9Semmelweis University, Department of Internal Medicine and Oncology, Budapest, Budapest, Hungary; 10Department of Surgery, Transplantation and Gastroenterology, Semmelweis University, Budapest, Budapest, Hungary; 11Central Hospital of Northern Pest- Military Hospital;, Budapest, Budapest, Hungary; 12East Lancashire Hospitals NHS Trust, Chorley, England, United Kingdom; 13Division of Gastroenterology and Hepatology, Feinberg School of Medicine, Northwestern University, Chicago, IL; 14Northern Care Alliance NHS Foundation Trust and University of Manchester, Manchester, England, United Kingdom
Introduction: The Vedolizumab Clinical Decision Support Tool (VDZ-CDST) has been demonstrated to predict clinical and endoscopic response to vedolizumab in Crohn’s disease (CD). Broader routine practice validation is needed to ensure generalizability across populations.
Methods: This retrospective multi-centre (7 centers, UK and Hungary) analysis included CD patients who received intravenous VDZ induction between 2015 and 2024. Available data were analysed without imputation of missing values. Patients were stratified using baseline pre-treatment information into low (≤13 points), intermediate ( >13 to ≤19 points), and high ( >19 points) probability of response groups. Clinical response (CRES), corticosteroid-free (CSF)-CRES, clinical remission (CREM) and CSF-CREM were assessed at the first visit, 6 months, and 12 months post-induction. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for the high/intermediate probability group (VDZ-CDST >13). Primary non-response (PNR), steroid sparing, IBD-related hospitalisation, and surgery were evaluated across all CDST strata.
Results: We included 231 patients [95 males; median age: 45.8 years {interquartile range (IQR): 29.1}]. CRES and CREM rates were incrementally lower from high to low probability groups across all timepoints (Table 1). The high-probability group had CSF-CREM rates of 58% at the first visit (median 3.4 months), 70% at 6 months, and 83% at 12 months, while the corresponding CSF-CREM rates in the low-probability group were 14%, 20%, and 21%, respectively. Using a cutoff of >13 (intermediate and high probability), sensitivity for CREM ranged from 93-97% with an NPV of 71-80%. Patients with high probability of response had greater steroid sparing (71% vs 39% in low group) and lower rates of IBD-related hospitalisation (12% vs 46%), PNR (10% vs 36%) and surgery (4% vs 32%) rates compared to the low probability cohort (Figure 1).
Discussion: VDZ-CDST effectively stratified CD patients by likelihood of VDZ response in real-world clinical practice. High-probability patients showed better outcomes across all measures, while low-probability patients had higher rates of PNR, hospitalisation, and surgery. VDZ-CDST’s high sensitivity and predictive value could be useful for identifying patients likely to benefit from VDZ therapy, thereby supporting clinical decision-making in IBD management.
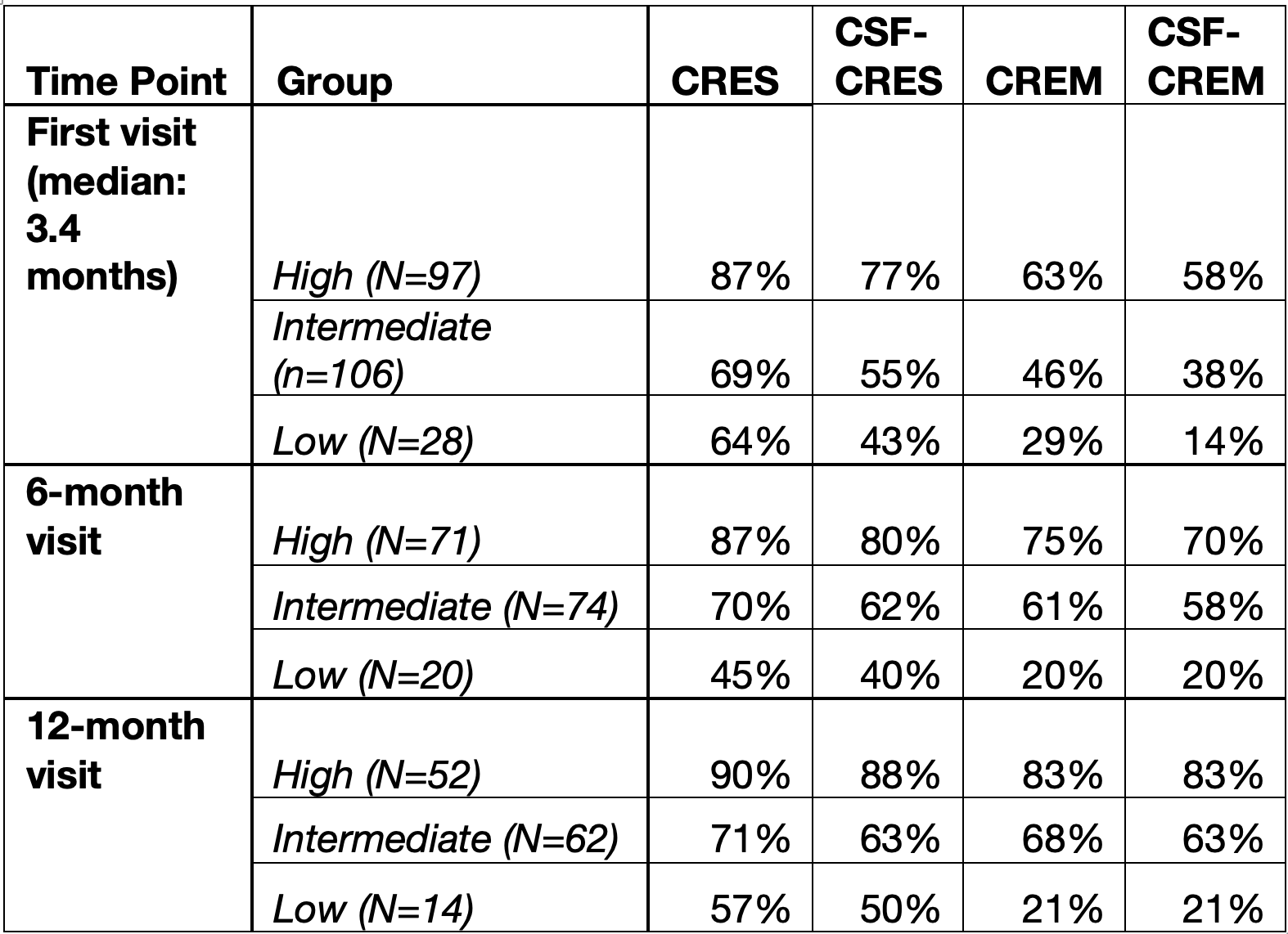
Figure: Table 1: Clinical Response and Remission Rates by VDZ-CDST Risk Category and Timepoint. CRES, clinical response; CREM, clinical remission; CSF, corticosteroid-free.
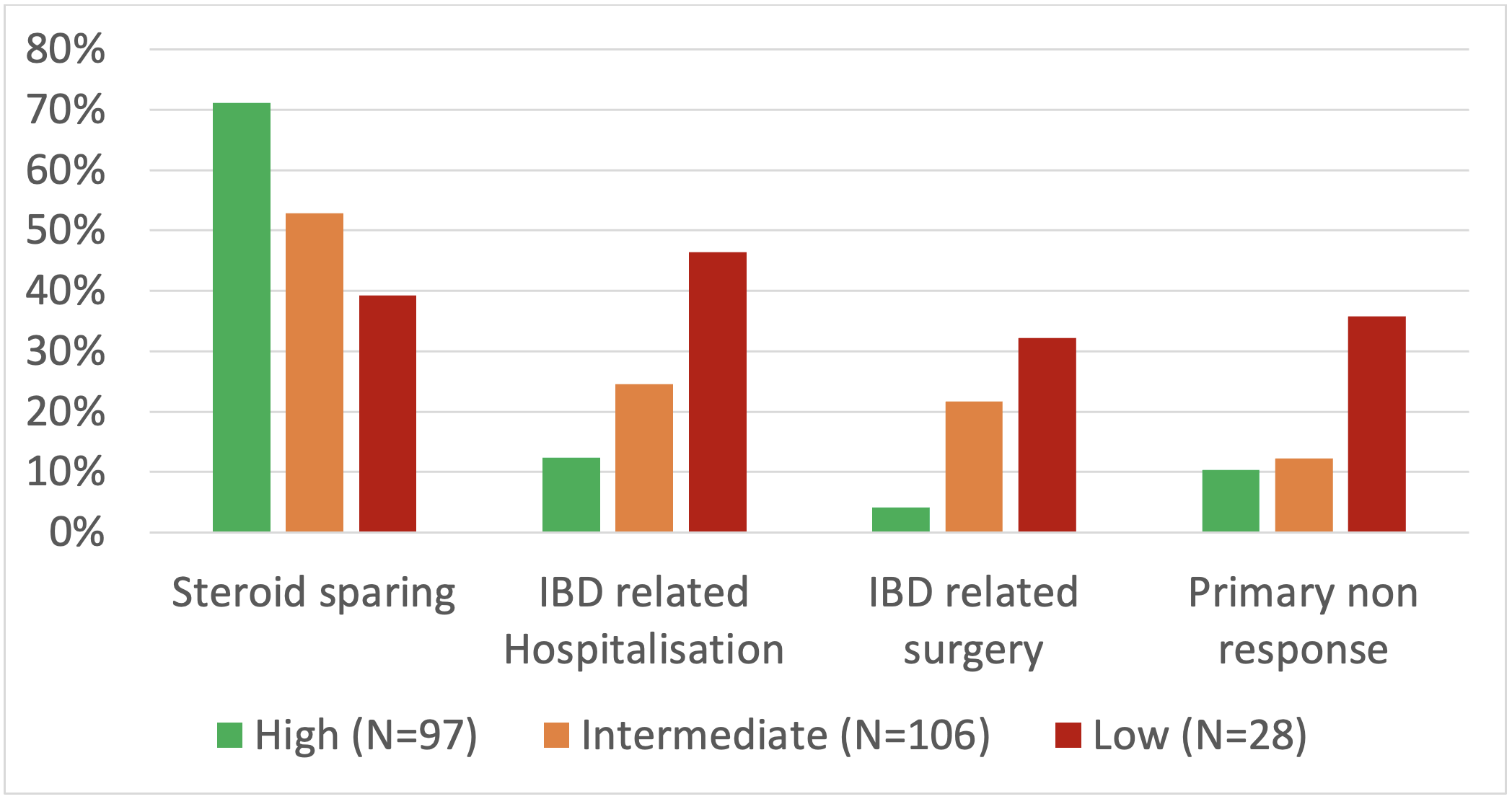
Figure: Figure 1: Clinical outcomes across the predicted patient groups
Disclosures:
Anish Kuriakose Kuzhiyanjal: DR Falk Pharma – Speakers Bureau. Takeda – Speakers Bureau.
Sarah Rhodes indicated no relevant financial relationships.
Bernadett Farkas indicated no relevant financial relationships.
Irene Latras-Cortés indicated no relevant financial relationships.
Emily Granger indicated no relevant financial relationships.
Stefanos Kateroglou indicated no relevant financial relationships.
Nzubechukwu Ozokwelu indicated no relevant financial relationships.
Usman Saleem Raza indicated no relevant financial relationships.
Emma Nixon indicated no relevant financial relationships.
Livia Lontai indicated no relevant financial relationships.
Orsolya Menyhárt indicated no relevant financial relationships.
Tibor Gyökeres: Astra-Zeneca – Advisory Committee/Board Member. Bristol Myers Sqibb – Advisory Committee/Board Member. Janssen-Cilag Kft – Advisory Committee/Board Member. Lilly Hungaria – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member. Phytotec – Advisory Committee/Board Member. Takeda Pharma – Advisory Committee/Board Member.
Mostafa Afifi indicated no relevant financial relationships.
Klaudia Farkas indicated no relevant financial relationships.
Tamás Molnár: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. BMS – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. EGIS – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. EwoPharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Fresenius – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Goodwill Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. MSD – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Phytotec – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Roche – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. SOBI – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. STADA – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. TEVA – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Parambir S. Dulai: AbbVie – Consultant. Abivax – Consultant. Adiso – Consultant. Alimentiv – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant. Digbi Health – Stock Options. Genentech – Consultant. Geneoscopy – Consultant. GSK – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant, Grant/Research Support. Precidiag – Royalties. Takeda – Consultant, Grant/Research Support.
Jimmy Limdi: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Abivax – Consultant, Speakers Bureau. AlfaSigma – Consultant, Speakers Bureau. Biohit – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau.
Anish Kuriakose Kuzhiyanjal, MBBS, MD, MRCP1, Sarah Rhodes, MSc, PhD2, Bernadett Farkas, MD3, Irene Latras-Cortés, MBBS, MD, MS4, Emily Granger, MBChB5, Stefanos Kateroglou, MD, MRCP1, Nzubechukwu Samuel. Ozokwelu, MBBS, MRCP6, Usman Saleem Raza, MBBS, MRCP7, Emma Nixon, MRCP8, Livia Lontai, MD9, Orsolya Menyhárt, MD10, Tibor Gyökeres, MD11, Mostafa I. Afifi, MBBCH, BSc012, Klaudia Farkas, MD, PhD3, Tamás Molnár, MD, PhD3, Parambir S. Dulai, MD13, Jimmy Limdi, MD14. P5313 - Real-World Utility of Vedolizumab Clinical Decision Support Tool for Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Award: ACG Presidential Poster Award
Anish Kuriakose Kuzhiyanjal, MBBS, MD, MRCP1, Sarah Rhodes, MSc, PhD2, Bernadett Farkas, MD3, Irene Latras-Cortés, MBBS, MD, MS4, Emily Granger, MBChB5, Stefanos Kateroglou, MD, MRCP1, Nzubechukwu Samuel. Ozokwelu, MBBS, MRCP6, Usman Saleem Raza, MBBS, MRCP7, Emma Nixon, MRCP8, Livia Lontai, MD9, Orsolya Menyhárt, MD10, Tibor Gyökeres, MD11, Mostafa I. Afifi, MBBCH, BSc012, Klaudia Farkas, MD, PhD3, Tamás Molnár, MD, PhD3, Parambir S. Dulai, MD13, Jimmy Limdi, MD14
1Northern Care Alliance NHS Foundation Trust, Manchester, England, United Kingdom; 2University of Manchester, Manchester, England, United Kingdom; 3University of Szeged, Szeged, Csongrad, Hungary; 4León University Hospital, León, Castilla y Leon, Spain; 5East Lancashire Hospitals NHS Trust, Blackburn, England, United Kingdom; 6Northern Care Alliance, Greater Manchester, England, United Kingdom; 7Salford Royal Hospital, Salford, England, United Kingdom; 8Northern Care alliance, Salford, England, United Kingdom; 9Semmelweis University, Department of Internal Medicine and Oncology, Budapest, Budapest, Hungary; 10Department of Surgery, Transplantation and Gastroenterology, Semmelweis University, Budapest, Budapest, Hungary; 11Central Hospital of Northern Pest- Military Hospital;, Budapest, Budapest, Hungary; 12East Lancashire Hospitals NHS Trust, Chorley, England, United Kingdom; 13Division of Gastroenterology and Hepatology, Feinberg School of Medicine, Northwestern University, Chicago, IL; 14Northern Care Alliance NHS Foundation Trust and University of Manchester, Manchester, England, United Kingdom
Introduction: The Vedolizumab Clinical Decision Support Tool (VDZ-CDST) has been demonstrated to predict clinical and endoscopic response to vedolizumab in Crohn’s disease (CD). Broader routine practice validation is needed to ensure generalizability across populations.
Methods: This retrospective multi-centre (7 centers, UK and Hungary) analysis included CD patients who received intravenous VDZ induction between 2015 and 2024. Available data were analysed without imputation of missing values. Patients were stratified using baseline pre-treatment information into low (≤13 points), intermediate ( >13 to ≤19 points), and high ( >19 points) probability of response groups. Clinical response (CRES), corticosteroid-free (CSF)-CRES, clinical remission (CREM) and CSF-CREM were assessed at the first visit, 6 months, and 12 months post-induction. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for the high/intermediate probability group (VDZ-CDST >13). Primary non-response (PNR), steroid sparing, IBD-related hospitalisation, and surgery were evaluated across all CDST strata.
Results: We included 231 patients [95 males; median age: 45.8 years {interquartile range (IQR): 29.1}]. CRES and CREM rates were incrementally lower from high to low probability groups across all timepoints (Table 1). The high-probability group had CSF-CREM rates of 58% at the first visit (median 3.4 months), 70% at 6 months, and 83% at 12 months, while the corresponding CSF-CREM rates in the low-probability group were 14%, 20%, and 21%, respectively. Using a cutoff of >13 (intermediate and high probability), sensitivity for CREM ranged from 93-97% with an NPV of 71-80%. Patients with high probability of response had greater steroid sparing (71% vs 39% in low group) and lower rates of IBD-related hospitalisation (12% vs 46%), PNR (10% vs 36%) and surgery (4% vs 32%) rates compared to the low probability cohort (Figure 1).
Discussion: VDZ-CDST effectively stratified CD patients by likelihood of VDZ response in real-world clinical practice. High-probability patients showed better outcomes across all measures, while low-probability patients had higher rates of PNR, hospitalisation, and surgery. VDZ-CDST’s high sensitivity and predictive value could be useful for identifying patients likely to benefit from VDZ therapy, thereby supporting clinical decision-making in IBD management.

Figure: Table 1: Clinical Response and Remission Rates by VDZ-CDST Risk Category and Timepoint. CRES, clinical response; CREM, clinical remission; CSF, corticosteroid-free.

Figure: Figure 1: Clinical outcomes across the predicted patient groups
Disclosures:
Anish Kuriakose Kuzhiyanjal: DR Falk Pharma – Speakers Bureau. Takeda – Speakers Bureau.
Sarah Rhodes indicated no relevant financial relationships.
Bernadett Farkas indicated no relevant financial relationships.
Irene Latras-Cortés indicated no relevant financial relationships.
Emily Granger indicated no relevant financial relationships.
Stefanos Kateroglou indicated no relevant financial relationships.
Nzubechukwu Ozokwelu indicated no relevant financial relationships.
Usman Saleem Raza indicated no relevant financial relationships.
Emma Nixon indicated no relevant financial relationships.
Livia Lontai indicated no relevant financial relationships.
Orsolya Menyhárt indicated no relevant financial relationships.
Tibor Gyökeres: Astra-Zeneca – Advisory Committee/Board Member. Bristol Myers Sqibb – Advisory Committee/Board Member. Janssen-Cilag Kft – Advisory Committee/Board Member. Lilly Hungaria – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member. Phytotec – Advisory Committee/Board Member. Takeda Pharma – Advisory Committee/Board Member.
Mostafa Afifi indicated no relevant financial relationships.
Klaudia Farkas indicated no relevant financial relationships.
Tamás Molnár: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. BMS – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. EGIS – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. EwoPharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Fresenius – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Goodwill Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. MSD – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Phytotec – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Roche – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. SOBI – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. STADA – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. TEVA – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Parambir S. Dulai: AbbVie – Consultant. Abivax – Consultant. Adiso – Consultant. Alimentiv – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant. Digbi Health – Stock Options. Genentech – Consultant. Geneoscopy – Consultant. GSK – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant, Grant/Research Support. Precidiag – Royalties. Takeda – Consultant, Grant/Research Support.
Jimmy Limdi: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Abivax – Consultant, Speakers Bureau. AlfaSigma – Consultant, Speakers Bureau. Biohit – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau.
Anish Kuriakose Kuzhiyanjal, MBBS, MD, MRCP1, Sarah Rhodes, MSc, PhD2, Bernadett Farkas, MD3, Irene Latras-Cortés, MBBS, MD, MS4, Emily Granger, MBChB5, Stefanos Kateroglou, MD, MRCP1, Nzubechukwu Samuel. Ozokwelu, MBBS, MRCP6, Usman Saleem Raza, MBBS, MRCP7, Emma Nixon, MRCP8, Livia Lontai, MD9, Orsolya Menyhárt, MD10, Tibor Gyökeres, MD11, Mostafa I. Afifi, MBBCH, BSc012, Klaudia Farkas, MD, PhD3, Tamás Molnár, MD, PhD3, Parambir S. Dulai, MD13, Jimmy Limdi, MD14. P5313 - Real-World Utility of Vedolizumab Clinical Decision Support Tool for Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

