Tuesday Poster Session
Category: IBD
P5304 - Heterogeneity in Randomized Controlled Trials Considering Fecal Microbiota Transplantation to Treat Ulcerative Colitis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Kanika Sehgal, MBBS (she/her/hers)
Yale New Haven Health
New Haven, CT
Presenting Author(s)
Kanika Sehgal, MBBS1, Alyssa Grimshaw, MBA, MPH, MSLIS2, Shanti Patel, MD1, Paul Feuerstadt, MD, FACG3
1Yale New Haven Health, New Haven, CT; 2Yale University School of Medicine, New Haven, CT; 3Yale University School of Medicine and PACT-Gastroenterology Center, Hamden, CT
Introduction: Significant heterogeneity of trial design is recognized in clinical trials evaluating fecal microbiota transplantation (FMT) as treatment for GI diseases. Multiple randomized controlled trials (RCTs) have attempted to assess the efficacy of FMT for treatment of ulcerative colitis (UC), however, the results have been variable. We hypothesize that RCTs considering FMT to treat UC suffer from heterogeneity of design and reporting.
Methods: A systematic literature search was conducted using Cochrane Library, Embase, Google Scholar, Medline, Scopus, and Web of Science from database inception through 4/16/25. We included all studies of adult populations with UC. 2 reviewers independently screened studies for inclusion and extracted study characteristics.
Results: 21 RCTs were identified matching our criteria (Table 1)(Figure 1). The majority of trials (19/21) investigated induction. Sample sizes varied, but were mostly < 100 (range 12-94). Most trials used the Mayo score to assess for severity and 3 used simple clinical colitis activity index (SCCAI). There was a large variability in the absolute grades of severity included across RCTs (Mayo scores range 4-10 and SCCAI scores range >5 to 3-9). The number of donors varied amongst trials (range 1-19); 2 had a single donor. Most used a local donor while 3 utilized a donor bank (2 used a domestic bank and 1 Openbiome). 11 used filtrate from a single donor and 7 used mixed donor samples (information was unclear in 5 trials). Variability in processing (12 frozen specimens, 6 fresh specimens, 1 oral capsules, 1 trial was unclear) and administration (colonoscopy was the initial route in 11 trials) were also seen. There was no codified dosing of formulations (only 1 study quantified the dose at 0·35 g of lyophilized stool per capsule, others did not provide any final dose measurements), and the number of times FMT was administered also varied widely across RCTs (range 1-288 doses). The control arm was also diverse, varying from placebo (n=8), autologous stool transplantation (n=4), antibiotics (n=1) or standard medical therapy (n=5). Follow-up duration was also highly variable (range 1-18 months).
Discussion: Amongst RCTs assessing the utility of FMT for UC, there was significant variation study structure and reporting. Therefore, to draw broad conclusions from such trials is very challenging. More codified structures and reporting requirements could enhance the generalizability of results.
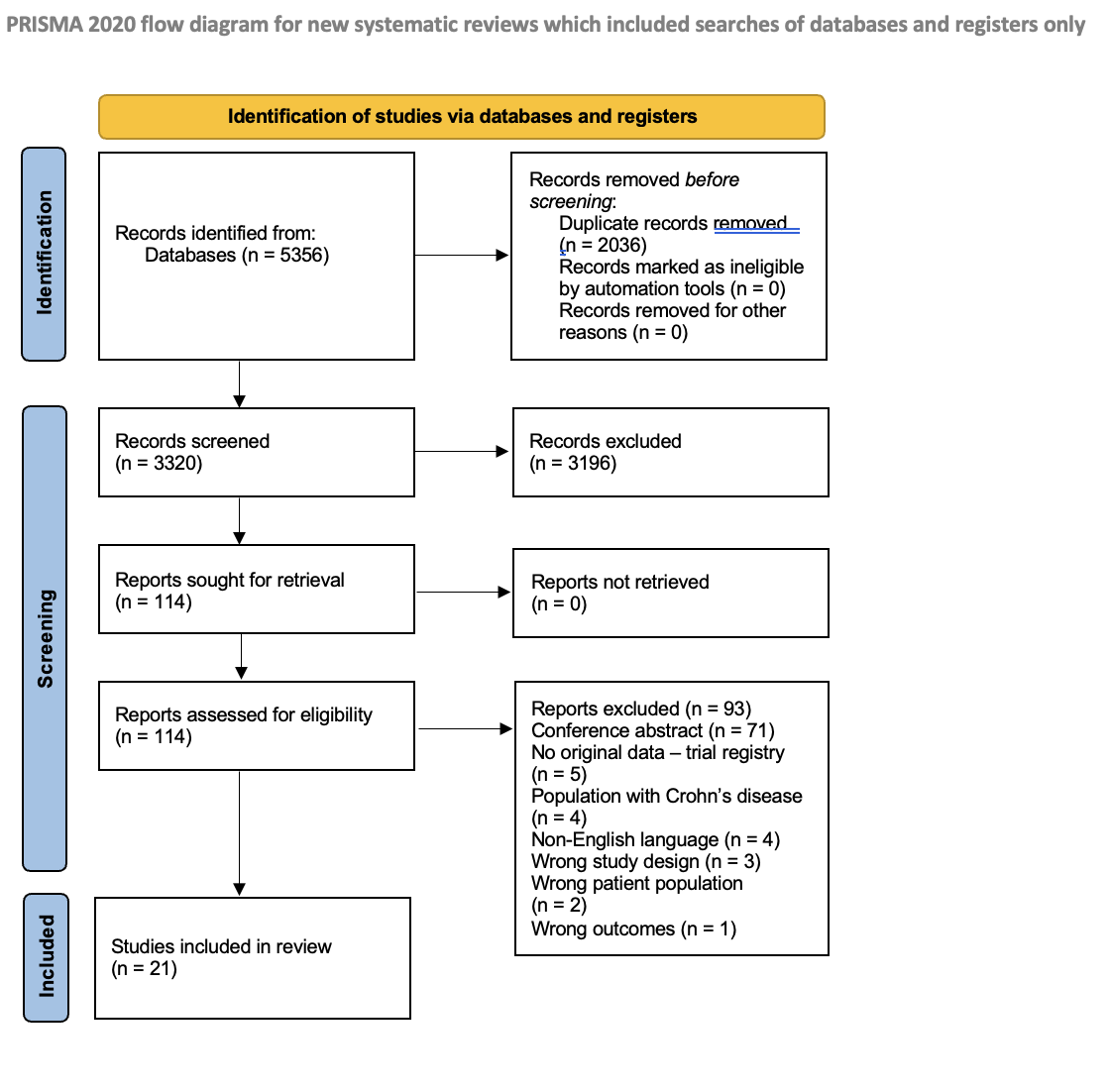
Figure: PRISMA flow diagram of studies included for systematic review
(From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71
For more information, visit: https://www.prisma-statement.org/)
Disclosures:
Kanika Sehgal indicated no relevant financial relationships.
Alyssa Grimshaw indicated no relevant financial relationships.
Shanti Patel indicated no relevant financial relationships.
Paul Feuerstadt: Ferring Pharmaceuticals – Advisor or Review Panel Member, Honoraria. Rebiotix, Inc. – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Honoraria. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Honoraria.
Kanika Sehgal, MBBS1, Alyssa Grimshaw, MBA, MPH, MSLIS2, Shanti Patel, MD1, Paul Feuerstadt, MD, FACG3. P5304 - Heterogeneity in Randomized Controlled Trials Considering Fecal Microbiota Transplantation to Treat Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Yale New Haven Health, New Haven, CT; 2Yale University School of Medicine, New Haven, CT; 3Yale University School of Medicine and PACT-Gastroenterology Center, Hamden, CT
Introduction: Significant heterogeneity of trial design is recognized in clinical trials evaluating fecal microbiota transplantation (FMT) as treatment for GI diseases. Multiple randomized controlled trials (RCTs) have attempted to assess the efficacy of FMT for treatment of ulcerative colitis (UC), however, the results have been variable. We hypothesize that RCTs considering FMT to treat UC suffer from heterogeneity of design and reporting.
Methods: A systematic literature search was conducted using Cochrane Library, Embase, Google Scholar, Medline, Scopus, and Web of Science from database inception through 4/16/25. We included all studies of adult populations with UC. 2 reviewers independently screened studies for inclusion and extracted study characteristics.
Results: 21 RCTs were identified matching our criteria (Table 1)(Figure 1). The majority of trials (19/21) investigated induction. Sample sizes varied, but were mostly < 100 (range 12-94). Most trials used the Mayo score to assess for severity and 3 used simple clinical colitis activity index (SCCAI). There was a large variability in the absolute grades of severity included across RCTs (Mayo scores range 4-10 and SCCAI scores range >5 to 3-9). The number of donors varied amongst trials (range 1-19); 2 had a single donor. Most used a local donor while 3 utilized a donor bank (2 used a domestic bank and 1 Openbiome). 11 used filtrate from a single donor and 7 used mixed donor samples (information was unclear in 5 trials). Variability in processing (12 frozen specimens, 6 fresh specimens, 1 oral capsules, 1 trial was unclear) and administration (colonoscopy was the initial route in 11 trials) were also seen. There was no codified dosing of formulations (only 1 study quantified the dose at 0·35 g of lyophilized stool per capsule, others did not provide any final dose measurements), and the number of times FMT was administered also varied widely across RCTs (range 1-288 doses). The control arm was also diverse, varying from placebo (n=8), autologous stool transplantation (n=4), antibiotics (n=1) or standard medical therapy (n=5). Follow-up duration was also highly variable (range 1-18 months).
Discussion: Amongst RCTs assessing the utility of FMT for UC, there was significant variation study structure and reporting. Therefore, to draw broad conclusions from such trials is very challenging. More codified structures and reporting requirements could enhance the generalizability of results.

Figure: PRISMA flow diagram of studies included for systematic review
(From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71
For more information, visit: https://www.prisma-statement.org/)
Disclosures:
Kanika Sehgal indicated no relevant financial relationships.
Alyssa Grimshaw indicated no relevant financial relationships.
Shanti Patel indicated no relevant financial relationships.
Paul Feuerstadt: Ferring Pharmaceuticals – Advisor or Review Panel Member, Honoraria. Rebiotix, Inc. – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Honoraria. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Honoraria.
Kanika Sehgal, MBBS1, Alyssa Grimshaw, MBA, MPH, MSLIS2, Shanti Patel, MD1, Paul Feuerstadt, MD, FACG3. P5304 - Heterogeneity in Randomized Controlled Trials Considering Fecal Microbiota Transplantation to Treat Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
