Tuesday Poster Session
Category: Functional Bowel Disease
P5097 - Plecanatide Is Efficacious in Women With Irritable Bowel Syndrome with Constipation (IBS-C) and Bloating: A Pooled Analysis of Two Phase 3, Randomized, Placebo-Controlled Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Darren M. Brenner, MD, FACG (he/him/his)
Professor of Medicine and Surgery
Northwestern University Feinberg School of Medicine, Chicago, IL, US
Chicago, IL
Presenting Author(s)
Darren M.. Brenner, MD1, Leila Neshatian, MD2, Felicia Shaya, DMSc3, Adam P.. Laitman, MD3, Dariush Shahsavari, MD4
1Northwestern University Feinberg School of Medicine, Chicago, IL, US, Chicago, IL; 2Stanford University, Stanford, CA; 3Salix Pharmaceuticals, Bridgewater, NJ; 4Emory University, Atlanta, GA
Introduction: Patients with IBS-C have abdominal symptoms, including pain and bloating, that prompt medical care. Thus, it is important to address and improve these 2 symptoms in addition to bowel movement (BM) frequency. IBS is more common in women, particularly those aged < 40 y. Thus, the aim was to evaluate plecanatide in young adult women with IBS-C with bloating using a trisymptom composite endpoint (abdominal pain, bloating, and complete spontaneous BM [CSBM] frequency) at various response thresholds.
Methods: Two phase 3 trials were conducted in adults with IBS-C randomized to plecanatide 3 mg, 6 mg, or placebo once daily for 12 wks. Abdominal pain (0 [“no pain”] to 10 [“worst possible pain”]), bloating (0 [“no bloating”] to 10 [“worst possible bloating”]), and CSBM occurrence were recorded daily. Data for females aged 18-40 y with any baseline bloating (score ≥ 1) were pooled and analyzed post hoc. Response was defined as simultaneous improvement from baseline in all 3 symptoms (abdominal pain, bloating, and CSBM/wk) for ≥ 6 of 12 wks using several composite criteria (ie, ≥ 2-point [or ≥ 30% or ≥ 40%] improvement in both abdominal pain and bloating plus a ≥ 1 [or ≥ 2] CSBM increase in the same wk for ≥ 6 of 12 wks). Data were further stratified by baseline bloating intensity (mild [score, 1-5]; moderate/severe [score, 6-10]).
Results: 651 females (median age, 31.0 y) with IBS-C and bloating (71.3% moderate/severe) were included (plecanatide 3 mg [n = 220]; 6 mg [n = 218]; placebo [n = 213]). Plecanatide 3 mg, 6 mg, and placebo group baseline mean symptom scores were 6.2, 6.3, and 6.4 for abdominal pain and 6.4, 6.5, and 6.7 for bloating, and the mean number of CSBMs/wk were 0.2, 0.3, and 0.2. Overall, a statistically significantly greater percentage of patients treated with plecanatide 3 mg or 6 mg vs placebo were trisymptom composite responders, defined using several stringent thresholds (Figure). When stratified by baseline bloating intensity, significant improvements favoring plecanatide (both doses) vs placebo were noted for most of the trisymptom composite endpoints (Table). Plecanatide was well tolerated.
Discussion: In women (aged 18-40 y) with IBS-C and bloating, plecanatide simultaneously and significantly improved abdominal pain, bloating, and CSBM frequency at multiple thresholds. Responses were maintained regardless of baseline bloating intensity. Plecanatide appears efficacious for treating abdominal and bowel symptoms characteristic of IBS-C in this patient population.
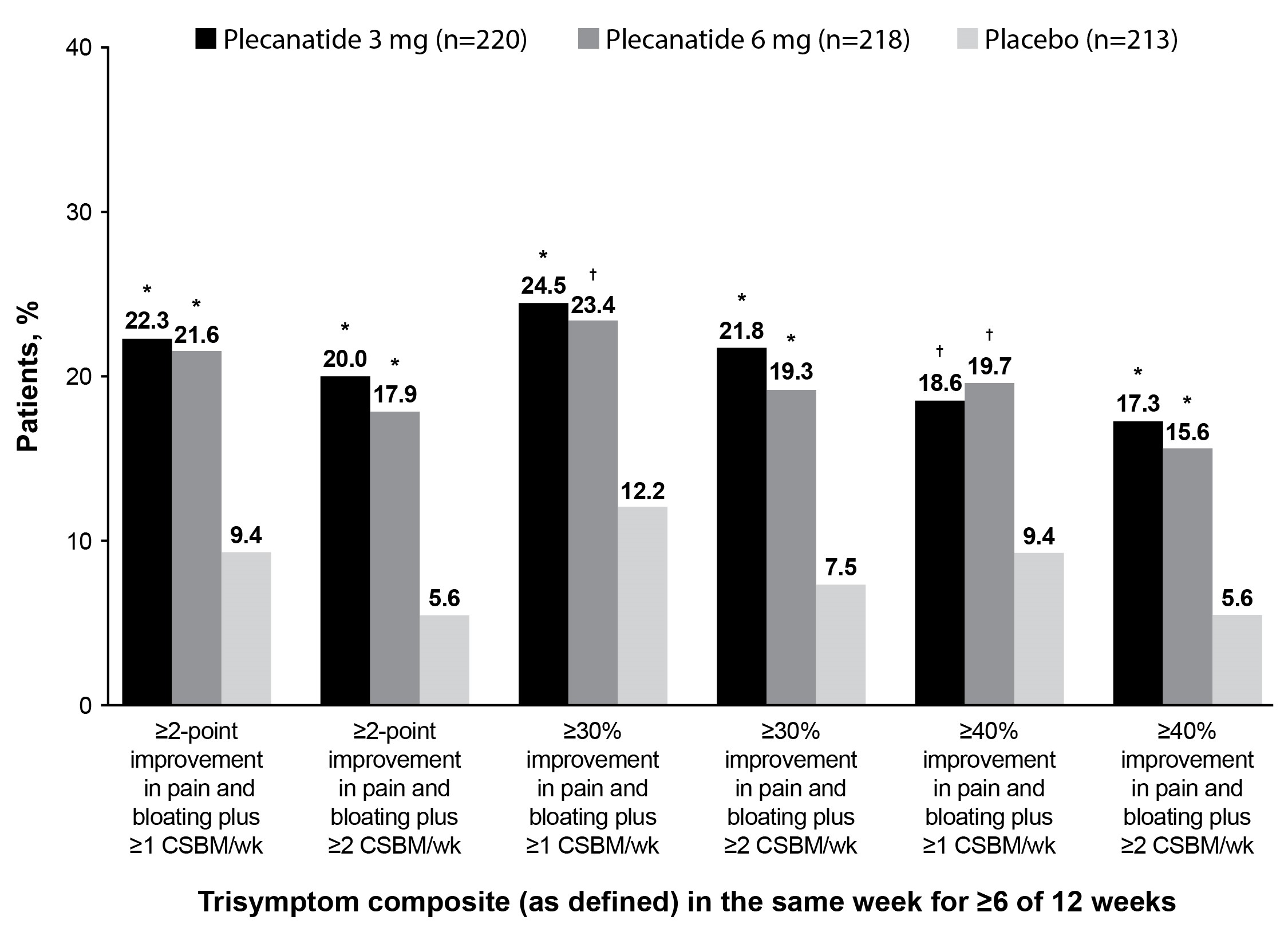
Figure: Figure. Trisymptom Composite Responders in Women Aged 18-40 Years With IBS-C and Baseline Bloating
*P < 0.001 vs placebo. †P < 0.01 vs placebo.
CSBM = complete spontaneous bowel movement; IBS-C = irritable bowel syndrome with constipation.
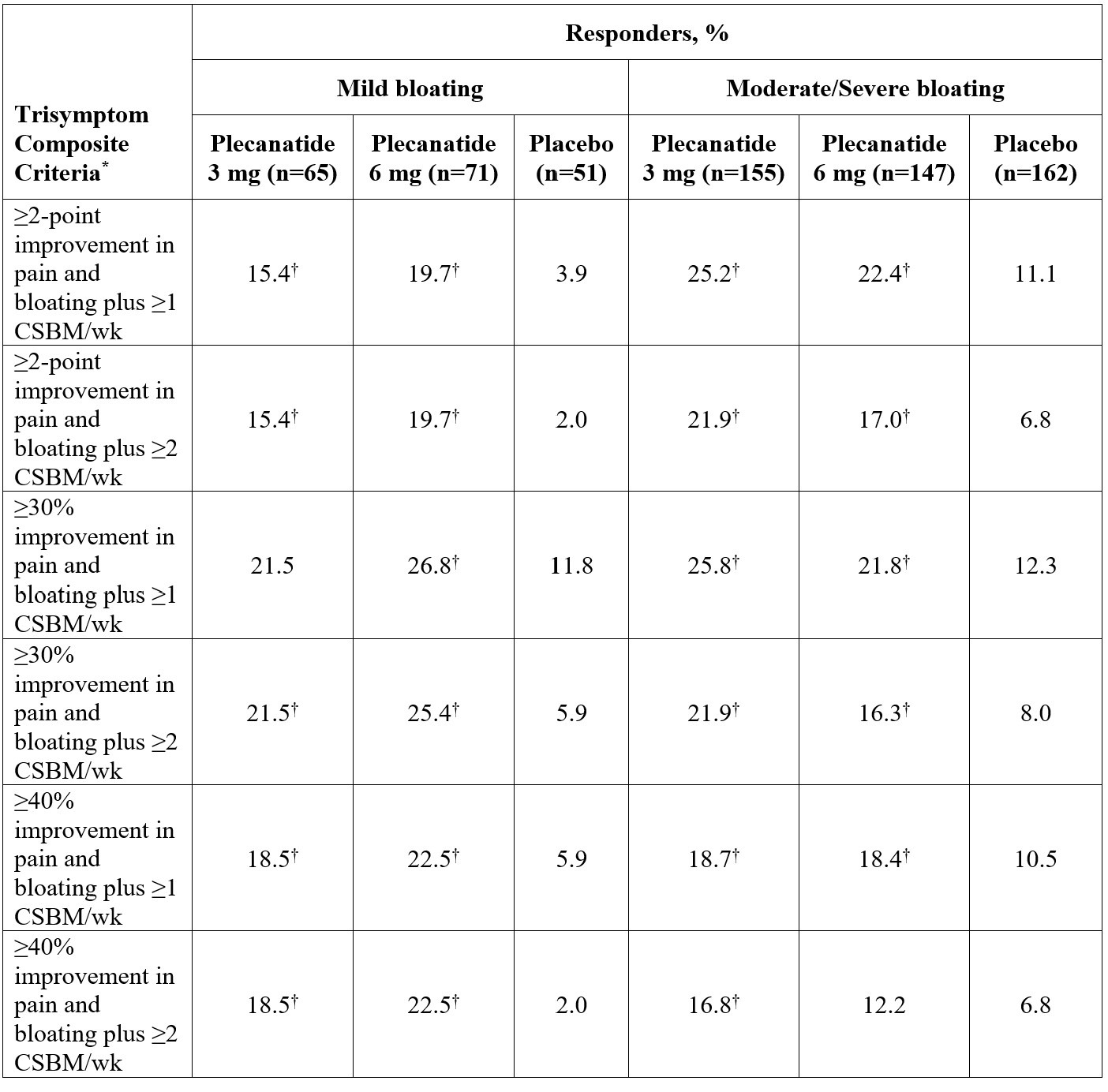
Figure: Table. Trisymptom Composite Responders in Women Aged 18-40 Years With IBS-C and Baseline Bloating, by Bloating Intensity
*Simultaneous (same wk) improvement from baseline, as defined, for ≥ 6 of 12 treatment wks.
†P < 0.05 vs placebo.
CSBM = complete spontaneous bowel movement; IBS-C = irritable bowel syndrome with constipation.
Disclosures:
Darren Brenner: Alnylam Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Anji Pharma, Ardelyx – Advisor or Review Panel Member. Ardelyx, AbbVie, Ironwood Pharmaceuticals, Bayer, Blueprint Medicines, CinPhloro Pharma, Dr Reddy’s Laboratories, Gemelli Biotech, Laborie – Consultant. Ardelyx, AbbVie, Ironwood Pharmaceuticals, Salix Pharmaceuticals – Speakers Bureau. Entrinsic Bioscience – Advisor or Review Panel Member, Consultant, Speaker. International Foundation for GI Disorders – Advisory Committee/Board Member. Mahana Therapeutics, Owlstone Medical, Salix Pharmaceuticals, Vibrant Pharma – Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Vibrant Gastro – Advisor or Review Panel Member, Consultant, Speaker.
Leila Neshatian indicated no relevant financial relationships.
Felicia Shaya: Salix Pharmaceuticals – Employee.
Adam Laitman: Salix Pharmaceuticals – Employee.
Dariush Shahsavari indicated no relevant financial relationships.
Darren M.. Brenner, MD1, Leila Neshatian, MD2, Felicia Shaya, DMSc3, Adam P.. Laitman, MD3, Dariush Shahsavari, MD4. P5097 - Plecanatide Is Efficacious in Women With Irritable Bowel Syndrome with Constipation (IBS-C) and Bloating: A Pooled Analysis of Two Phase 3, Randomized, Placebo-Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Northwestern University Feinberg School of Medicine, Chicago, IL, US, Chicago, IL; 2Stanford University, Stanford, CA; 3Salix Pharmaceuticals, Bridgewater, NJ; 4Emory University, Atlanta, GA
Introduction: Patients with IBS-C have abdominal symptoms, including pain and bloating, that prompt medical care. Thus, it is important to address and improve these 2 symptoms in addition to bowel movement (BM) frequency. IBS is more common in women, particularly those aged < 40 y. Thus, the aim was to evaluate plecanatide in young adult women with IBS-C with bloating using a trisymptom composite endpoint (abdominal pain, bloating, and complete spontaneous BM [CSBM] frequency) at various response thresholds.
Methods: Two phase 3 trials were conducted in adults with IBS-C randomized to plecanatide 3 mg, 6 mg, or placebo once daily for 12 wks. Abdominal pain (0 [“no pain”] to 10 [“worst possible pain”]), bloating (0 [“no bloating”] to 10 [“worst possible bloating”]), and CSBM occurrence were recorded daily. Data for females aged 18-40 y with any baseline bloating (score ≥ 1) were pooled and analyzed post hoc. Response was defined as simultaneous improvement from baseline in all 3 symptoms (abdominal pain, bloating, and CSBM/wk) for ≥ 6 of 12 wks using several composite criteria (ie, ≥ 2-point [or ≥ 30% or ≥ 40%] improvement in both abdominal pain and bloating plus a ≥ 1 [or ≥ 2] CSBM increase in the same wk for ≥ 6 of 12 wks). Data were further stratified by baseline bloating intensity (mild [score, 1-5]; moderate/severe [score, 6-10]).
Results: 651 females (median age, 31.0 y) with IBS-C and bloating (71.3% moderate/severe) were included (plecanatide 3 mg [n = 220]; 6 mg [n = 218]; placebo [n = 213]). Plecanatide 3 mg, 6 mg, and placebo group baseline mean symptom scores were 6.2, 6.3, and 6.4 for abdominal pain and 6.4, 6.5, and 6.7 for bloating, and the mean number of CSBMs/wk were 0.2, 0.3, and 0.2. Overall, a statistically significantly greater percentage of patients treated with plecanatide 3 mg or 6 mg vs placebo were trisymptom composite responders, defined using several stringent thresholds (Figure). When stratified by baseline bloating intensity, significant improvements favoring plecanatide (both doses) vs placebo were noted for most of the trisymptom composite endpoints (Table). Plecanatide was well tolerated.
Discussion: In women (aged 18-40 y) with IBS-C and bloating, plecanatide simultaneously and significantly improved abdominal pain, bloating, and CSBM frequency at multiple thresholds. Responses were maintained regardless of baseline bloating intensity. Plecanatide appears efficacious for treating abdominal and bowel symptoms characteristic of IBS-C in this patient population.

Figure: Figure. Trisymptom Composite Responders in Women Aged 18-40 Years With IBS-C and Baseline Bloating
*P < 0.001 vs placebo. †P < 0.01 vs placebo.
CSBM = complete spontaneous bowel movement; IBS-C = irritable bowel syndrome with constipation.

Figure: Table. Trisymptom Composite Responders in Women Aged 18-40 Years With IBS-C and Baseline Bloating, by Bloating Intensity
*Simultaneous (same wk) improvement from baseline, as defined, for ≥ 6 of 12 treatment wks.
†P < 0.05 vs placebo.
CSBM = complete spontaneous bowel movement; IBS-C = irritable bowel syndrome with constipation.
Disclosures:
Darren Brenner: Alnylam Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Anji Pharma, Ardelyx – Advisor or Review Panel Member. Ardelyx, AbbVie, Ironwood Pharmaceuticals, Bayer, Blueprint Medicines, CinPhloro Pharma, Dr Reddy’s Laboratories, Gemelli Biotech, Laborie – Consultant. Ardelyx, AbbVie, Ironwood Pharmaceuticals, Salix Pharmaceuticals – Speakers Bureau. Entrinsic Bioscience – Advisor or Review Panel Member, Consultant, Speaker. International Foundation for GI Disorders – Advisory Committee/Board Member. Mahana Therapeutics, Owlstone Medical, Salix Pharmaceuticals, Vibrant Pharma – Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Vibrant Gastro – Advisor or Review Panel Member, Consultant, Speaker.
Leila Neshatian indicated no relevant financial relationships.
Felicia Shaya: Salix Pharmaceuticals – Employee.
Adam Laitman: Salix Pharmaceuticals – Employee.
Dariush Shahsavari indicated no relevant financial relationships.
Darren M.. Brenner, MD1, Leila Neshatian, MD2, Felicia Shaya, DMSc3, Adam P.. Laitman, MD3, Dariush Shahsavari, MD4. P5097 - Plecanatide Is Efficacious in Women With Irritable Bowel Syndrome with Constipation (IBS-C) and Bloating: A Pooled Analysis of Two Phase 3, Randomized, Placebo-Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
