Tuesday Poster Session
Category: Functional Bowel Disease
P5083 - Real-World Safety Analysis of the Constipation-Predominant Irritable Bowel Syndrome Treatment Options Linaclotide, Lubiprostone, Plecanatide, and Tenapanor
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Michael B. Andrews, MD (he/him/his)
Virginia Commonwealth University Health System
Richmond, VA
Presenting Author(s)
Michael B. Andrews, MD1, Douglas G. Adler, MD, FACG2
1Virginia Commonwealth University Health System, Richmond, VA; 2Center for Advanced Therapeutic (CATE), Centura Health, Porter Adventist Hospital, Peak Gastroenterology, Denver, CO
Introduction: Linaclotide, Lubiprostone, and Plecanatide are FDA approved secretagogues for treatment of constipation-predominant irritable bowel syndrome (IBS-C). Tenapanor is the only retainagogue with FDA approval for IBS-C. Post-marketing adverse events (AEs) associated with the use of each medication are submitted by healthcare professionals, drug manufacturers, and consumers to the FDA Adverse Event Reporting System (FAERS) database.
Methods: All reports from each medication’s FDA approval date until June 30, 2024 were downloaded from the publicly available FAERS database. Reports with a reason for use outside of IBS and/or constipation and reports with other suspected medications listed were excluded from analysis. After exclusion criteria were applied, analysis was performed on 5,787 Linaclotide reports, 670 Lubiprostone reports, 368 Plecanatide reports, and 122 Tenapanor reports. Many reports contained multiple suspected medication-associated AEs.
Results: Linaclotide was suspected of frequently inducing diarrhea (n=2,082, 24.1%), abdominal pain (n=815, 9.4%), abdominal bloating (n=795, 9.2%), and nausea/vomiting (n=266, 3.1%). Plecanatide was suspected of frequently inducing diarrhea (n=137, 20.4%), abdominal pain (n=76, 11.3%), abdominal bloating (n=62, 9.2%), and nausea/vomiting (n=34, 5.1%). Tenapanor was suspected of frequently inducing diarrhea (n=51, 32.9%), abdominal pain (n=13, 8.4%), and abdominal bloating and nausea/vomiting (n=11 each, 7.1%). Lubiprostone was suspected of frequently inducing dyspnea (n=221, 13.0%), nausea/vomiting (n=161, 9.5%), chest pain (n=157, 9.3%), abdominal pain (n=85, 5.0%), and diarrhea (n=79, 4.7%).
Discussion: This real-world safety analysis of post-marketing data from FAERS identified diarrhea, abdominal pain, bloating, and nausea/vomiting as the most reported AEs across all FDA approved IBS-C medications. Most notably, Linaclotide showed the potential to induce clinically significant dehydration, and Lubiprostone showed the potential to induce dyspnea and chest pain. While causation cannot be implied due to the limitations of FAERS, these findings are important to stimulate future safety studies and enhance shared-decision making between providers and patients with IBS-C.
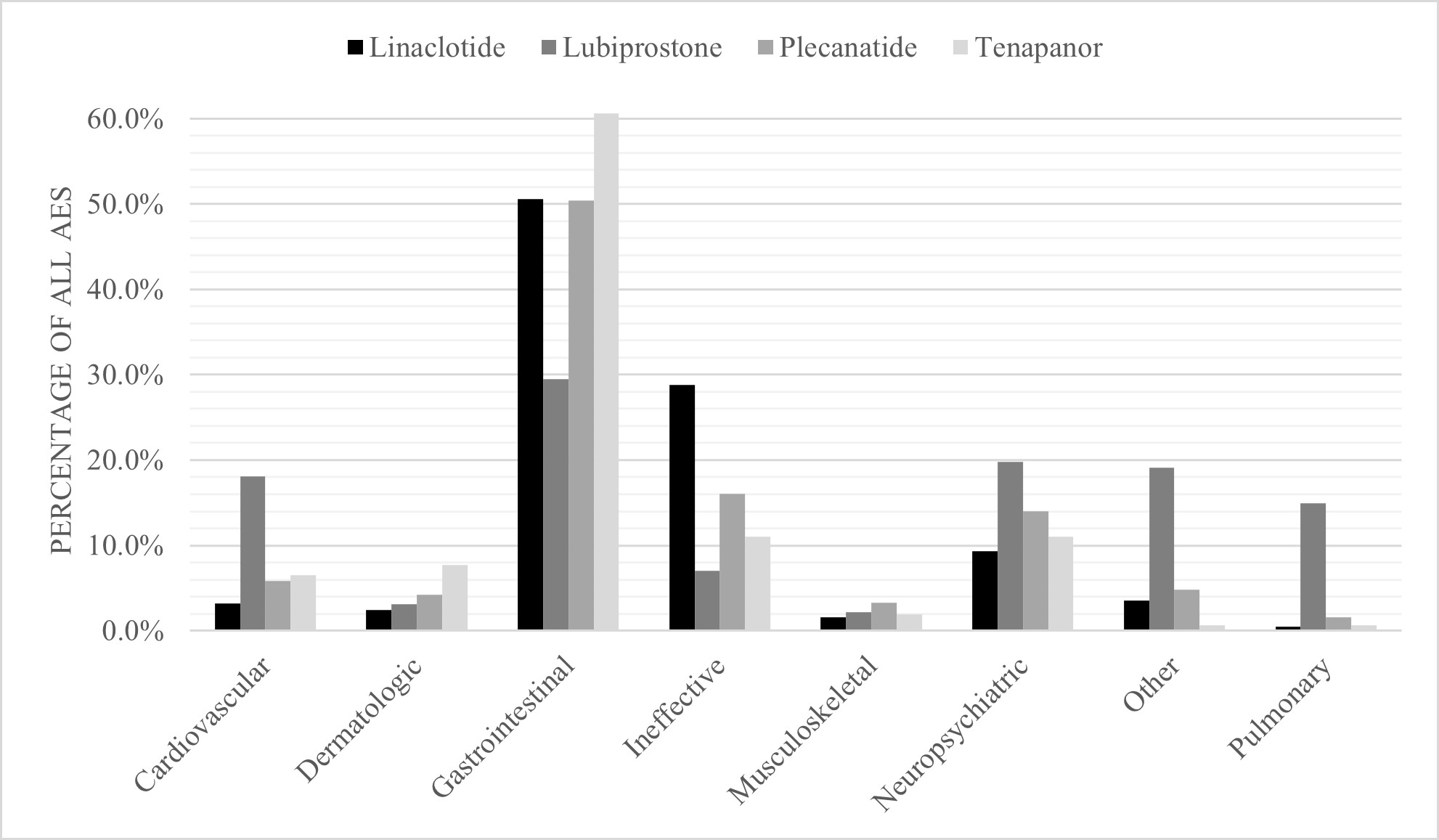
Figure: Figure 1. AEs by Organ System
Disclosures:
Michael Andrews indicated no relevant financial relationships.
Douglas Adler: Boston Scientific – Consultant.
Michael B. Andrews, MD1, Douglas G. Adler, MD, FACG2. P5083 - Real-World Safety Analysis of the Constipation-Predominant Irritable Bowel Syndrome Treatment Options Linaclotide, Lubiprostone, Plecanatide, and Tenapanor, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Virginia Commonwealth University Health System, Richmond, VA; 2Center for Advanced Therapeutic (CATE), Centura Health, Porter Adventist Hospital, Peak Gastroenterology, Denver, CO
Introduction: Linaclotide, Lubiprostone, and Plecanatide are FDA approved secretagogues for treatment of constipation-predominant irritable bowel syndrome (IBS-C). Tenapanor is the only retainagogue with FDA approval for IBS-C. Post-marketing adverse events (AEs) associated with the use of each medication are submitted by healthcare professionals, drug manufacturers, and consumers to the FDA Adverse Event Reporting System (FAERS) database.
Methods: All reports from each medication’s FDA approval date until June 30, 2024 were downloaded from the publicly available FAERS database. Reports with a reason for use outside of IBS and/or constipation and reports with other suspected medications listed were excluded from analysis. After exclusion criteria were applied, analysis was performed on 5,787 Linaclotide reports, 670 Lubiprostone reports, 368 Plecanatide reports, and 122 Tenapanor reports. Many reports contained multiple suspected medication-associated AEs.
Results: Linaclotide was suspected of frequently inducing diarrhea (n=2,082, 24.1%), abdominal pain (n=815, 9.4%), abdominal bloating (n=795, 9.2%), and nausea/vomiting (n=266, 3.1%). Plecanatide was suspected of frequently inducing diarrhea (n=137, 20.4%), abdominal pain (n=76, 11.3%), abdominal bloating (n=62, 9.2%), and nausea/vomiting (n=34, 5.1%). Tenapanor was suspected of frequently inducing diarrhea (n=51, 32.9%), abdominal pain (n=13, 8.4%), and abdominal bloating and nausea/vomiting (n=11 each, 7.1%). Lubiprostone was suspected of frequently inducing dyspnea (n=221, 13.0%), nausea/vomiting (n=161, 9.5%), chest pain (n=157, 9.3%), abdominal pain (n=85, 5.0%), and diarrhea (n=79, 4.7%).
Discussion: This real-world safety analysis of post-marketing data from FAERS identified diarrhea, abdominal pain, bloating, and nausea/vomiting as the most reported AEs across all FDA approved IBS-C medications. Most notably, Linaclotide showed the potential to induce clinically significant dehydration, and Lubiprostone showed the potential to induce dyspnea and chest pain. While causation cannot be implied due to the limitations of FAERS, these findings are important to stimulate future safety studies and enhance shared-decision making between providers and patients with IBS-C.

Figure: Figure 1. AEs by Organ System
Disclosures:
Michael Andrews indicated no relevant financial relationships.
Douglas Adler: Boston Scientific – Consultant.
Michael B. Andrews, MD1, Douglas G. Adler, MD, FACG2. P5083 - Real-World Safety Analysis of the Constipation-Predominant Irritable Bowel Syndrome Treatment Options Linaclotide, Lubiprostone, Plecanatide, and Tenapanor, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
