Tuesday Poster Session
Category: Functional Bowel Disease
P5066 - A 2-Week Course of Rifaximin Improves Symptoms of Irritable Bowel Syndrome With Diarrhea (IBS-D) in Patients With Comorbid Anxiety
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- MP
Mark Pimentel, MD, FACG
Cedars-Sinai Medical Center
Los Angeles, CA
Presenting Author(s)
Mark Pimentel, MD, FACG1, Lucinda Harris, MD2, Ali Rezaie, MD, MSc1, Christopher Allen, MS3, Zeev Heimanson, PharmD3, Eric Shah, MD4
1Cedars-Sinai Medical Center, Los Angeles, CA; 2Mayo Clinic, Scottsdale, AZ; 3Salix Pharmaceuticals, Bridgewater, NJ; 4University of Michigan, Ann Arbor, MI
Introduction: Anxiety, a common comorbidity in patients with IBS-D, can negatively impact gastrointestinal symptoms. Data are limited on the potential impact of anxiety on medications targeting IBS-D symptoms. This post hoc analysis evaluated the efficacy of rifaximin, indicated for the treatment of adults with IBS-D, in individuals with comorbid anxiety.
Methods: Data were pooled from 2 identically designed, phase 3, randomized, double-blind trials. Adults with IBS-D were randomly assigned to receive rifaximin 550 mg or placebo 3 times daily for 2 weeks, followed by a 4-week treatment-free period to assess response, and an additional 6 weeks of follow-up. This population was stratified post hoc by medical history of anxiety. Abdominal pain/discomfort and bloating were separately rated daily (0 [“not at all”] to 6 [“a very great deal”]) and stool consistency was scored from 1 (“very hard”) to 5 (“watery”). Efficacy assessments included the percentage of patients with adequate global IBS symptom relief (“In regards to your IBS symptoms, compared to the way you felt before you started study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms?) and composite response (≥ 30% decrease from baseline in weekly mean abdominal pain/discomfort score plus a weekly mean stool consistency score < 4) during ≥ 2 of first 4 weeks post-treatment.
Results: A total of 232 patients had comorbid anxiety (rifaximin [n = 114]; placebo [n = 118]) and most were female (84.2%; 79.7%); the mean (SD) age was 45.0 (14.4) and 44.9 (13.7) years, respectively. Baseline scores in the rifaximin and placebo groups were similar for mean daily abdominal pain/discomfort (3.3; 3.2), bloating, (3.4; 3.3), and stool consistency (3.9; 3.9). A greater percentage of patients with comorbid anxiety treated with rifaximin had adequate relief of global IBS symptoms vs placebo (43.0% vs 28.0%; relative difference = 42.3%; absolute difference = 15.0%; P = 0.02) and were composite responders (46.5% vs 33.9%; relative difference = 31.3%; absolute difference=12.6%; P = 0.05; Fig. 1). Significantly greater improvements from baseline in abdominal pain/discomfort were observed for rifaximin vs placebo at most timepoints through 10 weeks of post-treatment follow-up (P < 0.05; Fig. 2).
Discussion: A 2-week course of rifaximin demonstrated improvements in symptoms of IBS-D compared with placebo in adults with comorbid anxiety, further supporting its potential as a treatment option for this patient population.
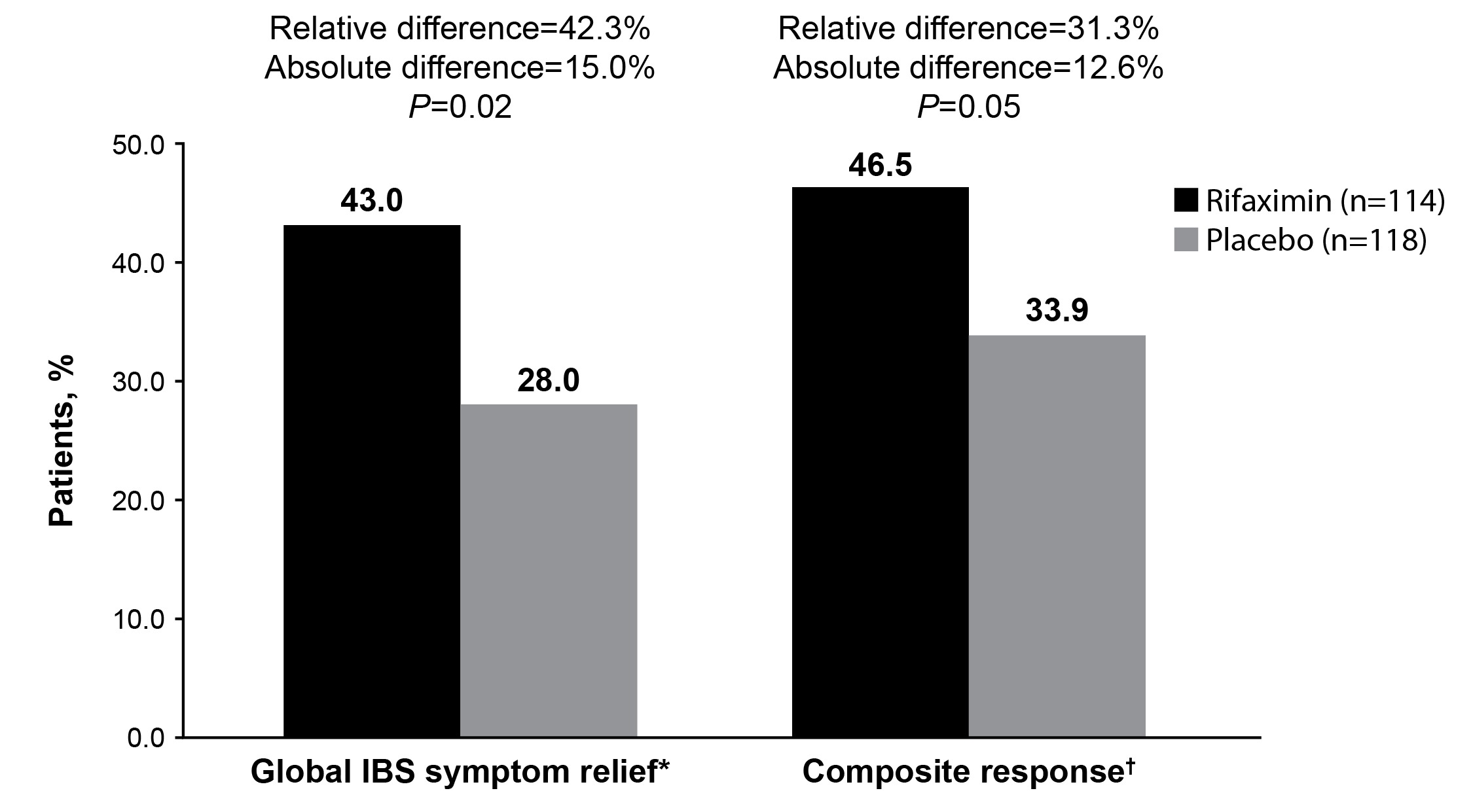
Figure: Fig. 1. Percentage of Patients With IBS-D and Comorbid Anxiety Achieving Adequate Relief of Global IBS Symptoms and Composite Response During ≥ 2 of First 4 Weeks Post-Treatment
*"In regards to your IBS symptoms, compared to the way you felt before you started study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms?"
†≥ 30% decrease from baseline in weekly mean abdominal pain/discomfort score plus a weekly mean stool consistency score < 4.
IBS = irritable bowel syndrome ; IBS-D = irritable bowel syndrome with diarrhea.
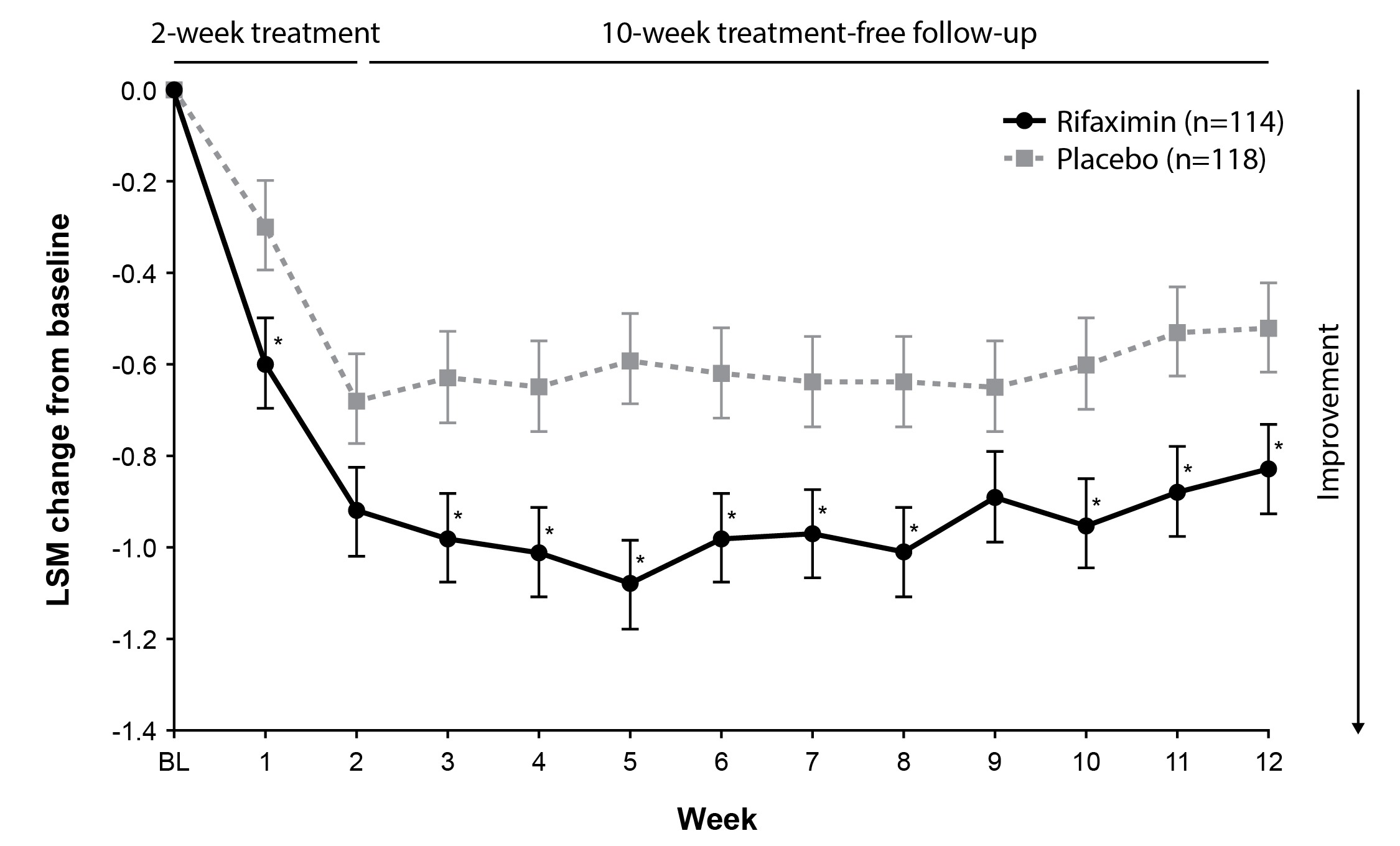
Figure: Fig. 2. Change From Baseline in Abdominal Pain/Discomfort Scores Over Time in Patients With IBS-D and Comorbid Anxiety
*P < 0.05 vs placebo.
BL= baseline; IBS-D = irritable bowel syndrome with diarrhea; LSM = least-squares mean.
Disclosures:
Mark Pimentel: Ardelyx – Consultant. Gemelli Biotech – Advisory Committee/Board Member, Royalties, Stock-privately held company. GoodLFE – Stock-privately held company. Salvo Health, Cylinder Health – Stock Options.
Lucinda Harris: Anyx – Grant/Research Support. Ardelyx – Consultant, Educational video. Gemelli Biotech – Advisory Committee/Board Member, Consultant. GI Health Foundation – Advisor or Review Panel Member. Rome – Member. Salix Pharmaceuticals – Consultant. Takeda – Grant/Research Support.
Ali Rezaie: Ardelyx, Blueprint Medicine and Salix Pharmaceuticals – Consultant. Gemelli Biotech, and Good LFE – Equity stake.
Christopher Allen: Salix Pharmaceuticals – Employee.
Zeev Heimanson: Salix Pharmaceuticals – Employee.
Eric Shah: Salix Pharmaceuticals – Consultant.
Mark Pimentel, MD, FACG1, Lucinda Harris, MD2, Ali Rezaie, MD, MSc1, Christopher Allen, MS3, Zeev Heimanson, PharmD3, Eric Shah, MD4. P5066 - A 2-Week Course of Rifaximin Improves Symptoms of Irritable Bowel Syndrome With Diarrhea (IBS-D) in Patients With Comorbid Anxiety, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cedars-Sinai Medical Center, Los Angeles, CA; 2Mayo Clinic, Scottsdale, AZ; 3Salix Pharmaceuticals, Bridgewater, NJ; 4University of Michigan, Ann Arbor, MI
Introduction: Anxiety, a common comorbidity in patients with IBS-D, can negatively impact gastrointestinal symptoms. Data are limited on the potential impact of anxiety on medications targeting IBS-D symptoms. This post hoc analysis evaluated the efficacy of rifaximin, indicated for the treatment of adults with IBS-D, in individuals with comorbid anxiety.
Methods: Data were pooled from 2 identically designed, phase 3, randomized, double-blind trials. Adults with IBS-D were randomly assigned to receive rifaximin 550 mg or placebo 3 times daily for 2 weeks, followed by a 4-week treatment-free period to assess response, and an additional 6 weeks of follow-up. This population was stratified post hoc by medical history of anxiety. Abdominal pain/discomfort and bloating were separately rated daily (0 [“not at all”] to 6 [“a very great deal”]) and stool consistency was scored from 1 (“very hard”) to 5 (“watery”). Efficacy assessments included the percentage of patients with adequate global IBS symptom relief (“In regards to your IBS symptoms, compared to the way you felt before you started study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms?) and composite response (≥ 30% decrease from baseline in weekly mean abdominal pain/discomfort score plus a weekly mean stool consistency score < 4) during ≥ 2 of first 4 weeks post-treatment.
Results: A total of 232 patients had comorbid anxiety (rifaximin [n = 114]; placebo [n = 118]) and most were female (84.2%; 79.7%); the mean (SD) age was 45.0 (14.4) and 44.9 (13.7) years, respectively. Baseline scores in the rifaximin and placebo groups were similar for mean daily abdominal pain/discomfort (3.3; 3.2), bloating, (3.4; 3.3), and stool consistency (3.9; 3.9). A greater percentage of patients with comorbid anxiety treated with rifaximin had adequate relief of global IBS symptoms vs placebo (43.0% vs 28.0%; relative difference = 42.3%; absolute difference = 15.0%; P = 0.02) and were composite responders (46.5% vs 33.9%; relative difference = 31.3%; absolute difference=12.6%; P = 0.05; Fig. 1). Significantly greater improvements from baseline in abdominal pain/discomfort were observed for rifaximin vs placebo at most timepoints through 10 weeks of post-treatment follow-up (P < 0.05; Fig. 2).
Discussion: A 2-week course of rifaximin demonstrated improvements in symptoms of IBS-D compared with placebo in adults with comorbid anxiety, further supporting its potential as a treatment option for this patient population.

Figure: Fig. 1. Percentage of Patients With IBS-D and Comorbid Anxiety Achieving Adequate Relief of Global IBS Symptoms and Composite Response During ≥ 2 of First 4 Weeks Post-Treatment
*"In regards to your IBS symptoms, compared to the way you felt before you started study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms?"
†≥ 30% decrease from baseline in weekly mean abdominal pain/discomfort score plus a weekly mean stool consistency score < 4.
IBS = irritable bowel syndrome ; IBS-D = irritable bowel syndrome with diarrhea.

Figure: Fig. 2. Change From Baseline in Abdominal Pain/Discomfort Scores Over Time in Patients With IBS-D and Comorbid Anxiety
*P < 0.05 vs placebo.
BL= baseline; IBS-D = irritable bowel syndrome with diarrhea; LSM = least-squares mean.
Disclosures:
Mark Pimentel: Ardelyx – Consultant. Gemelli Biotech – Advisory Committee/Board Member, Royalties, Stock-privately held company. GoodLFE – Stock-privately held company. Salvo Health, Cylinder Health – Stock Options.
Lucinda Harris: Anyx – Grant/Research Support. Ardelyx – Consultant, Educational video. Gemelli Biotech – Advisory Committee/Board Member, Consultant. GI Health Foundation – Advisor or Review Panel Member. Rome – Member. Salix Pharmaceuticals – Consultant. Takeda – Grant/Research Support.
Ali Rezaie: Ardelyx, Blueprint Medicine and Salix Pharmaceuticals – Consultant. Gemelli Biotech, and Good LFE – Equity stake.
Christopher Allen: Salix Pharmaceuticals – Employee.
Zeev Heimanson: Salix Pharmaceuticals – Employee.
Eric Shah: Salix Pharmaceuticals – Consultant.
Mark Pimentel, MD, FACG1, Lucinda Harris, MD2, Ali Rezaie, MD, MSc1, Christopher Allen, MS3, Zeev Heimanson, PharmD3, Eric Shah, MD4. P5066 - A 2-Week Course of Rifaximin Improves Symptoms of Irritable Bowel Syndrome With Diarrhea (IBS-D) in Patients With Comorbid Anxiety, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
