Tuesday Poster Session
Category: Functional Bowel Disease
P5062 - Treatment Satisfaction with Tenapanor (IBSRELA): Real-World Survey of Patients With Irritable Bowel Syndrome With Constipation
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- LS
Luisa Scott, PhD
Ardelyx, Inc.
Waltham, MA
Presenting Author(s)
Luisa Scott, PhD1, Johannah Ruddy, M.Ed1, Alice Sibelli, PhD2, Belinda Gist, 1, Laura Williams, MD, MPH1, Tedra D. Gray, NP3, Subhankar Chakraborty, MD, PhD4
1Ardelyx, Inc., Waltham, MA; 2SideBy Care, Redesign Health, London, England, United Kingdom; 3Sinai Chicago, Chicago, IL; 4The Ohio State University Wexner Medical Center, Columbus, OH
Introduction: Irritable bowel syndrome with constipation (IBS-C) is a common disorder of gut-brain interaction, characterized by abdominal pain, bloating, and constipation, that negatively impact patients’ quality of life (QoL). Tenapanor (TEN [IBSRELA]) is a sodium/hydrogen exchanger 3 inhibitor indicated for treatment of IBS-C in adults. Real-world data on TEN regarding treatment satisfaction, IBS-C symptom resolution, and improvement in QoL are lacking. Herein, we report results of a survey of patients receiving TEN for the treatment of IBS-C, which was conducted to address these data gaps.
Methods: The survey was distributed via text messaging through the ArdelyxAssist patient assistance program (PAP). Patients were aged ≥18 years and actively receiving TEN—first dispensed ≥6.5 weeks prior and last dispensed within 90 days before the survey start. Multifactor ordered logistic regression analysis was performed to determine predictors of QoL improvements and treatment satisfaction.
Results: Of the 4252 invites sent, 537 patients completed the survey. As shown in the Figure, most patients reported treatment satisfaction with TEN (88%) as well as improved constipation (95%), bloating (75%), and abdominal pain (84%). All 3 symptoms improved in 69% of patients. QoL also improved with TEN, with patients reporting that TEN improved their ability to participate in daily activities, including work (74%) or social activities and exercise (74%). Improvements in constipation, bloating, and abdominal pain were each predictive of improved QoL (Figure D), while improvement in constipation was predictive of treatment satisfaction (OR, 3.19; P=0.02). In response to open-ended questions about the impact of TEN, 87% of patients shared positive experiences with TEN, and 76% shared that TEN is better than other IBS-C medications they have used. Thirty percent of patients shared challenges, including side effects and partial symptom resolution with < 3% mentioning that potential side effects impacted their QoL.
Discussion: Patients in the real world report treatment satisfaction and improvements in constipation, bloating, abdominal pain, and QoL with TEN. Patients expressed that TEN worked better and/or had fewer side effects than other medications. Overall, the findings highlight the effectiveness of TEN in the management of IBS-C.
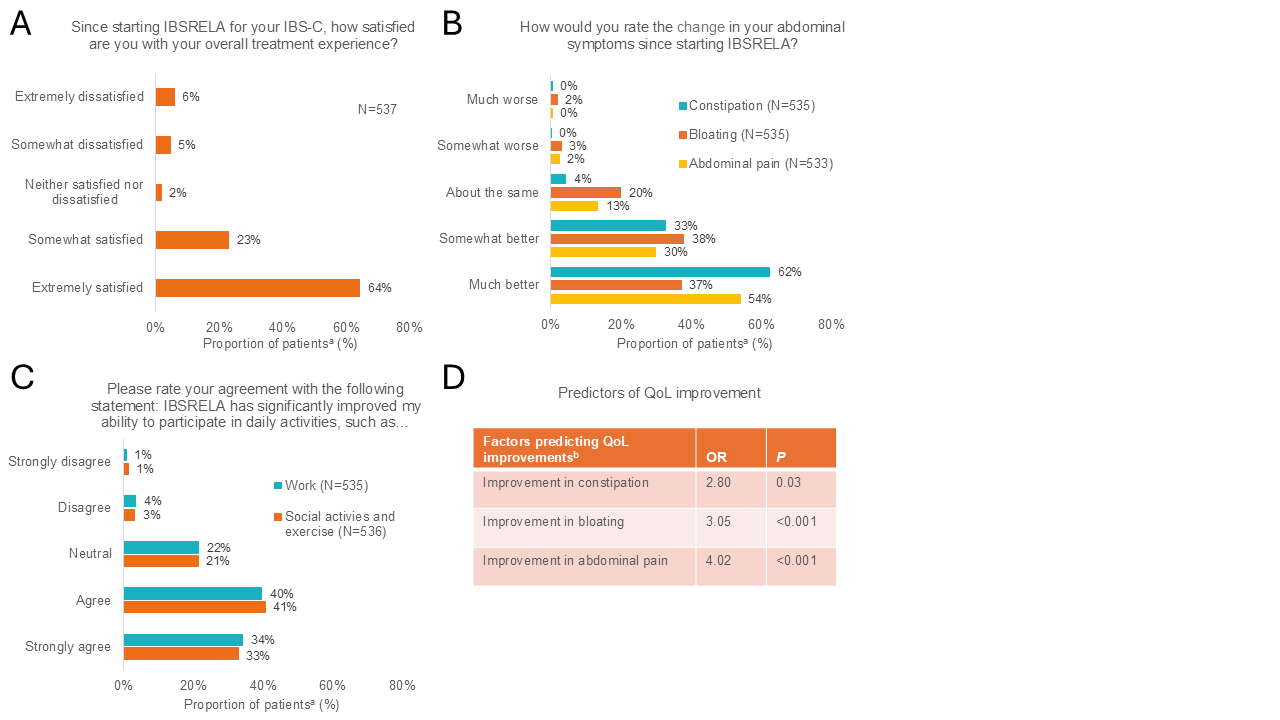
Figure: Figure: Patient Survey Responses on (A) Treatment Satisfaction, (B) Abdominal Symptoms, and (C) QoL. (D) Multifactor Regression Analysis to Determine Predictors of QoL Improvements
aAll data label percentages are rounded to the nearest integer.
bAll factors tested are included in table.
IBS-C, irritable bowel syndrome with constipation; OR, odds ratio; QoL, quality of life.
Disclosures:
Luisa Scott: Ardelyx, Inc. – Employee.
Johannah Ruddy: Ardelyx, Inc. – Employee.
Alice Sibelli: Ardelyx, Inc. – Consultant.
Belinda Gist: Ardelyx, Inc. – Employee.
Laura Williams: Ardelyx, Inc. – Employee.
Tedra Gray: Ardelyx, Inc. – Advisory Committee/Board Member, Consultant. Castle Biosciences – Speakers Bureau. Phathom Pharmaceuticals – Speakers Bureau. Salix Pharmaceuticals – Advisory Committee/Board Member, Consultant. Sanofi/Regeneron – Speakers Bureau.
Subhankar Chakraborty: Ardelyx, Inc. – Grant/Research Support. Calmigo – Grant/Research Support. Medtronic – Grant/Research Support. Proppr – Grant/Research Support. Regeneron – Grant/Research Support. Soothing Scents – Grant/Research Support. Vanda Pharmaceuticals – Grant/Research Support. Wat Medical – Grant/Research Support.
Luisa Scott, PhD1, Johannah Ruddy, M.Ed1, Alice Sibelli, PhD2, Belinda Gist, 1, Laura Williams, MD, MPH1, Tedra D. Gray, NP3, Subhankar Chakraborty, MD, PhD4. P5062 - Treatment Satisfaction with Tenapanor (IBSRELA): Real-World Survey of Patients With Irritable Bowel Syndrome With Constipation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Ardelyx, Inc., Waltham, MA; 2SideBy Care, Redesign Health, London, England, United Kingdom; 3Sinai Chicago, Chicago, IL; 4The Ohio State University Wexner Medical Center, Columbus, OH
Introduction: Irritable bowel syndrome with constipation (IBS-C) is a common disorder of gut-brain interaction, characterized by abdominal pain, bloating, and constipation, that negatively impact patients’ quality of life (QoL). Tenapanor (TEN [IBSRELA]) is a sodium/hydrogen exchanger 3 inhibitor indicated for treatment of IBS-C in adults. Real-world data on TEN regarding treatment satisfaction, IBS-C symptom resolution, and improvement in QoL are lacking. Herein, we report results of a survey of patients receiving TEN for the treatment of IBS-C, which was conducted to address these data gaps.
Methods: The survey was distributed via text messaging through the ArdelyxAssist patient assistance program (PAP). Patients were aged ≥18 years and actively receiving TEN—first dispensed ≥6.5 weeks prior and last dispensed within 90 days before the survey start. Multifactor ordered logistic regression analysis was performed to determine predictors of QoL improvements and treatment satisfaction.
Results: Of the 4252 invites sent, 537 patients completed the survey. As shown in the Figure, most patients reported treatment satisfaction with TEN (88%) as well as improved constipation (95%), bloating (75%), and abdominal pain (84%). All 3 symptoms improved in 69% of patients. QoL also improved with TEN, with patients reporting that TEN improved their ability to participate in daily activities, including work (74%) or social activities and exercise (74%). Improvements in constipation, bloating, and abdominal pain were each predictive of improved QoL (Figure D), while improvement in constipation was predictive of treatment satisfaction (OR, 3.19; P=0.02). In response to open-ended questions about the impact of TEN, 87% of patients shared positive experiences with TEN, and 76% shared that TEN is better than other IBS-C medications they have used. Thirty percent of patients shared challenges, including side effects and partial symptom resolution with < 3% mentioning that potential side effects impacted their QoL.
Discussion: Patients in the real world report treatment satisfaction and improvements in constipation, bloating, abdominal pain, and QoL with TEN. Patients expressed that TEN worked better and/or had fewer side effects than other medications. Overall, the findings highlight the effectiveness of TEN in the management of IBS-C.

Figure: Figure: Patient Survey Responses on (A) Treatment Satisfaction, (B) Abdominal Symptoms, and (C) QoL. (D) Multifactor Regression Analysis to Determine Predictors of QoL Improvements
aAll data label percentages are rounded to the nearest integer.
bAll factors tested are included in table.
IBS-C, irritable bowel syndrome with constipation; OR, odds ratio; QoL, quality of life.
Disclosures:
Luisa Scott: Ardelyx, Inc. – Employee.
Johannah Ruddy: Ardelyx, Inc. – Employee.
Alice Sibelli: Ardelyx, Inc. – Consultant.
Belinda Gist: Ardelyx, Inc. – Employee.
Laura Williams: Ardelyx, Inc. – Employee.
Tedra Gray: Ardelyx, Inc. – Advisory Committee/Board Member, Consultant. Castle Biosciences – Speakers Bureau. Phathom Pharmaceuticals – Speakers Bureau. Salix Pharmaceuticals – Advisory Committee/Board Member, Consultant. Sanofi/Regeneron – Speakers Bureau.
Subhankar Chakraborty: Ardelyx, Inc. – Grant/Research Support. Calmigo – Grant/Research Support. Medtronic – Grant/Research Support. Proppr – Grant/Research Support. Regeneron – Grant/Research Support. Soothing Scents – Grant/Research Support. Vanda Pharmaceuticals – Grant/Research Support. Wat Medical – Grant/Research Support.
Luisa Scott, PhD1, Johannah Ruddy, M.Ed1, Alice Sibelli, PhD2, Belinda Gist, 1, Laura Williams, MD, MPH1, Tedra D. Gray, NP3, Subhankar Chakraborty, MD, PhD4. P5062 - Treatment Satisfaction with Tenapanor (IBSRELA): Real-World Survey of Patients With Irritable Bowel Syndrome With Constipation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
