Tuesday Poster Session
Category: Functional Bowel Disease
P5061 - Transcutaneous Auricular Vagus Nerve Stimulation Shows Promising Symptom Improvement in Functional Gastrointestinal Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Ashesh Das, MBBS
KPC Medical College and Hospital , Kolkata, India
Kolkata, West Bengal, India
Presenting Author(s)
Ashesh Das, MBBS1, Sunaisha Addanki, MBBS2, Aarushi Gupta, 3, Venkata Dileep Kumar Veldi, MBBS4, Rohit Baidya, 5, Nihal Reddy, MBBS6, Shayan Mahapatra, MD7, Anveshak , 8, Gowrishankar Palaniswamy, MBBS9, Muhammad Ashar Khan, MBBS10, Saketh Mehul Echampati, MBBS11, Saketh Vinjamuri, MBBS12
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Gandhi Medical College & Hospital, Secunderabad, India, Hyderabad, Telangana, India; 3Avalon University School of Medicine, Irvine, CA; 4Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 5Institute of Post-Graduate Medical Education and Research and Seth Sukhlal Karnani Memorial Hospital, Kolkata, West Bengal, India; 6ESIC Medical College and Hospital Sanathnagar, Hyderabad, Telangana, India; 7Aiken Regional Medical Center, Graniteville, SC; 8Hassan Institute of Medical Sciences, Bengaluru, Karnataka, India; 9Medical University of South Carolina, Lancaster, SC; 10Liaquat College of Medicine and Dentistry, Karachi, Sindh, Pakistan; 11SRM Medical College Hospital and Research Centre, Hyderabad, Telangana, India; 12Gandhi Medical College and Hospital, Secunderabad, Telangana, India, Santa Clara, CA
Introduction: Transcutaneous auricular vagus-nerve stimulation (taVNS) is a needle-free neuromodulation strategy that may normalise gut–brain signalling in functional gastrointestinal disorders (FGIDs). No quantitative synthesis has yet evaluated its efficacy or safety. We therefore performed the first meta-analysis of randomized evidence to clarify taVNS’s efficacy and safety across functional dyspepsia (FD), Irritable Bowel Syndrome Type C (IBS-C), and chronic constipation (CC).
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified Randomized Controlled Trials (RCTs) comparing taVNS Vs SHAM in to treat various functional GI disorders through March 2025.Efficacy calculations used the per-protocol counts reported by Shi et al. (189 taVNS, 95 sham), whereas Adverse Event (AEs) analyses retained the full ITT populations across all trials (272 vs 174 participants). Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Three RCTs (IBS-C n = 40; CC n = 106; FD two-dose trial n = 284) contributed 261 taVNS and 169 sham patients. The functional-dyspepsia RCT evaluated two active taVNS doses (V10 and V25). Responder rates were 70.1 % (183/261) versus 34.9 % (59/169); pooled RR 1.98 (95 % CI 0.85-4.61, p = 0.12). Substantial heterogeneity was detected (I² = 75 %), largely attributable to the neutral chronic-constipation trial; benefit was marked in IBS-C (RR 8.5) and FD (RR 1.68). Absolute risk reduction 35.2 % yields an NNT of 3. Safety was reassuring: AEs occurred in 8.5 % vs 12.1 % (23/272 vs 21/174), RR 1.01 (0.59-1.73), I² = 0 %. No serious device-related events were found.
Discussion: taVNS emerged as a safe, non-invasive therapeutic option that consistently improved symptom severity and global response rates in irritable bowel syndrome-constipation and functional dyspepsia. However, variability driven by a neutral chronic-constipation trial warrants the need for larger, disorder-specific RCTs with harmonized stimulation protocols, objective biomarkers, and long-term follow-up to confirm clinical durability and its potential for inclusion in the guidelines.
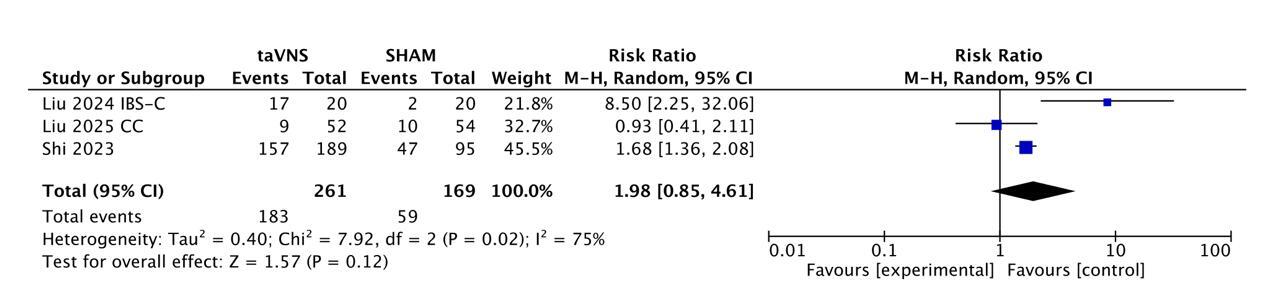
Figure: Figure- Forest Plot showing Responders at 4 Weeks
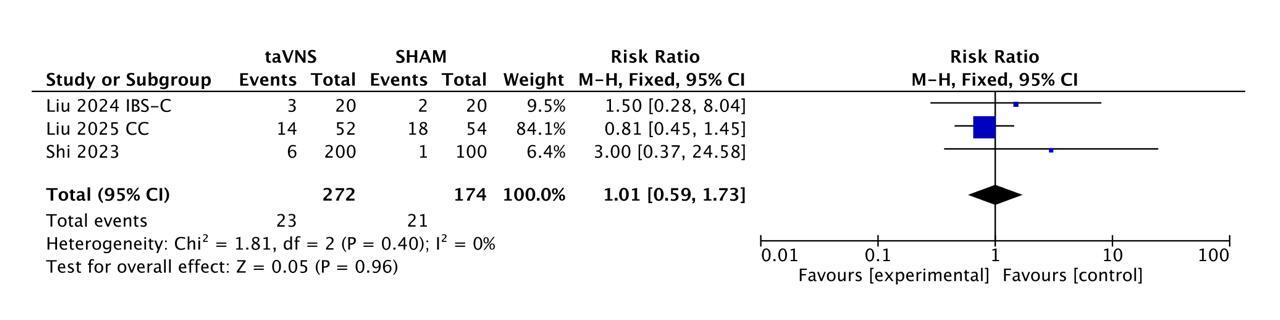
Figure: Figure - Forest Plot showing Safety Profile
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Sunaisha Addanki indicated no relevant financial relationships.
Aarushi Gupta indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Rohit Baidya indicated no relevant financial relationships.
Nihal Reddy indicated no relevant financial relationships.
Shayan Mahapatra indicated no relevant financial relationships.
Anveshak indicated no relevant financial relationships.
Gowrishankar Palaniswamy indicated no relevant financial relationships.
Muhammad Ashar Khan indicated no relevant financial relationships.
Saketh Mehul Echampati indicated no relevant financial relationships.
Saketh Vinjamuri indicated no relevant financial relationships.
Ashesh Das, MBBS1, Sunaisha Addanki, MBBS2, Aarushi Gupta, 3, Venkata Dileep Kumar Veldi, MBBS4, Rohit Baidya, 5, Nihal Reddy, MBBS6, Shayan Mahapatra, MD7, Anveshak , 8, Gowrishankar Palaniswamy, MBBS9, Muhammad Ashar Khan, MBBS10, Saketh Mehul Echampati, MBBS11, Saketh Vinjamuri, MBBS12. P5061 - Transcutaneous Auricular Vagus Nerve Stimulation Shows Promising Symptom Improvement in Functional Gastrointestinal Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Gandhi Medical College & Hospital, Secunderabad, India, Hyderabad, Telangana, India; 3Avalon University School of Medicine, Irvine, CA; 4Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 5Institute of Post-Graduate Medical Education and Research and Seth Sukhlal Karnani Memorial Hospital, Kolkata, West Bengal, India; 6ESIC Medical College and Hospital Sanathnagar, Hyderabad, Telangana, India; 7Aiken Regional Medical Center, Graniteville, SC; 8Hassan Institute of Medical Sciences, Bengaluru, Karnataka, India; 9Medical University of South Carolina, Lancaster, SC; 10Liaquat College of Medicine and Dentistry, Karachi, Sindh, Pakistan; 11SRM Medical College Hospital and Research Centre, Hyderabad, Telangana, India; 12Gandhi Medical College and Hospital, Secunderabad, Telangana, India, Santa Clara, CA
Introduction: Transcutaneous auricular vagus-nerve stimulation (taVNS) is a needle-free neuromodulation strategy that may normalise gut–brain signalling in functional gastrointestinal disorders (FGIDs). No quantitative synthesis has yet evaluated its efficacy or safety. We therefore performed the first meta-analysis of randomized evidence to clarify taVNS’s efficacy and safety across functional dyspepsia (FD), Irritable Bowel Syndrome Type C (IBS-C), and chronic constipation (CC).
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified Randomized Controlled Trials (RCTs) comparing taVNS Vs SHAM in to treat various functional GI disorders through March 2025.Efficacy calculations used the per-protocol counts reported by Shi et al. (189 taVNS, 95 sham), whereas Adverse Event (AEs) analyses retained the full ITT populations across all trials (272 vs 174 participants). Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Three RCTs (IBS-C n = 40; CC n = 106; FD two-dose trial n = 284) contributed 261 taVNS and 169 sham patients. The functional-dyspepsia RCT evaluated two active taVNS doses (V10 and V25). Responder rates were 70.1 % (183/261) versus 34.9 % (59/169); pooled RR 1.98 (95 % CI 0.85-4.61, p = 0.12). Substantial heterogeneity was detected (I² = 75 %), largely attributable to the neutral chronic-constipation trial; benefit was marked in IBS-C (RR 8.5) and FD (RR 1.68). Absolute risk reduction 35.2 % yields an NNT of 3. Safety was reassuring: AEs occurred in 8.5 % vs 12.1 % (23/272 vs 21/174), RR 1.01 (0.59-1.73), I² = 0 %. No serious device-related events were found.
Discussion: taVNS emerged as a safe, non-invasive therapeutic option that consistently improved symptom severity and global response rates in irritable bowel syndrome-constipation and functional dyspepsia. However, variability driven by a neutral chronic-constipation trial warrants the need for larger, disorder-specific RCTs with harmonized stimulation protocols, objective biomarkers, and long-term follow-up to confirm clinical durability and its potential for inclusion in the guidelines.

Figure: Figure- Forest Plot showing Responders at 4 Weeks

Figure: Figure - Forest Plot showing Safety Profile
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Sunaisha Addanki indicated no relevant financial relationships.
Aarushi Gupta indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Rohit Baidya indicated no relevant financial relationships.
Nihal Reddy indicated no relevant financial relationships.
Shayan Mahapatra indicated no relevant financial relationships.
Anveshak indicated no relevant financial relationships.
Gowrishankar Palaniswamy indicated no relevant financial relationships.
Muhammad Ashar Khan indicated no relevant financial relationships.
Saketh Mehul Echampati indicated no relevant financial relationships.
Saketh Vinjamuri indicated no relevant financial relationships.
Ashesh Das, MBBS1, Sunaisha Addanki, MBBS2, Aarushi Gupta, 3, Venkata Dileep Kumar Veldi, MBBS4, Rohit Baidya, 5, Nihal Reddy, MBBS6, Shayan Mahapatra, MD7, Anveshak , 8, Gowrishankar Palaniswamy, MBBS9, Muhammad Ashar Khan, MBBS10, Saketh Mehul Echampati, MBBS11, Saketh Vinjamuri, MBBS12. P5061 - Transcutaneous Auricular Vagus Nerve Stimulation Shows Promising Symptom Improvement in Functional Gastrointestinal Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
