Tuesday Poster Session
Category: Esophagus
P5014 - Severe Esophagitis Induced by Durvalumab in the Treatment of Advanced Urothelial Cancer: A Case Report
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- MA
Michael Adams, DO (he/him/his)
Walter Reed National Military Medical Center
Bethesda, MD
Presenting Author(s)
Kelly M. Vo, DO, Nathaniel Goss, MD, Michael Adams, DO, Patrick Young, MD, FACG
Walter Reed National Military Medical Center, Bethesda, MD
Introduction: Durvalumab, an immune checkpoint inhibitor targeting PD-L1, shows promise in treating advanced urothelial cell cancer. Though generally well tolerated, it can lead to immune-related adverse events (irAEs). These have been observed in non-small cell lung cancer treatment but not, to date, in advanced urothelial cell bladder cancer. We present a case of severe esophagitis in a patient receiving durvalumab for advanced bladder cancer, underscoring the need for close monitoring.
Case Description/
Methods: A 58-year-old man with Stage IIIA (pT3aN0[0/39]M0) muscle-invasive bladder cancer (MIBC) with a predominant sarcomatoid urothelial variant and low-risk localized prostate cancer (pT2N0[0/39]) underwent open radical cystoprostatectomy, retroperitoneal lymph node dissection, and ileal conduit creation in August 2024. Three months later, he began neoadjuvant therapy with cisplatin, gemcitabine, and durvalumab, followed by adjuvant monotherapy with durvalumab. After two cycles of monotherapy, he developed severe dysphagia, nausea, and vomiting, 84 days after initiating chemotherapy. Hospital evaluation revealed stable vital signs and abdominal tenderness. A computed tomography scan showed distal esophageal thickening and gastric dilation (Figure 1). Esophagogastroduodenoscopy revealed severe esophagitis with mucosal damage (Figure 2). He was diagnosed with grade 3 ICI-induced esophagitis and treated with intravenous steroids, later transitioned to oral prednisone. His symptoms improved, and he was discharged on a steroid taper. Unfortunately, he died 25 days later; the cause of death remains unknown due to the absence of an autopsy.
Discussion: Durvalumab is FDA-approved for muscle-invasive Stage III urothelial cancer following cystectomy, with improved survival demonstrated in the NIAGARA trial. While gastrointestinal irAEs such as colitis are known, esophagitis is rare. In NSCLC, esophagitis occurred in 6.3% of patients post-chemoradiotherapy, typically during durvalumab treatment. The timing in this case aligns with previously reported patterns, and symptoms responded to glucocorticoids. Gemcitabine and cisplatin are not typically associated with esophagitis unless used with radiation. This is the first documented case of esophagitis related to durvalumab in urothelial cancer. Although the patient improved in hospital, the cause of his death post-discharge remains unclear but is likely unrelated to the irAE given his clinical improvement.
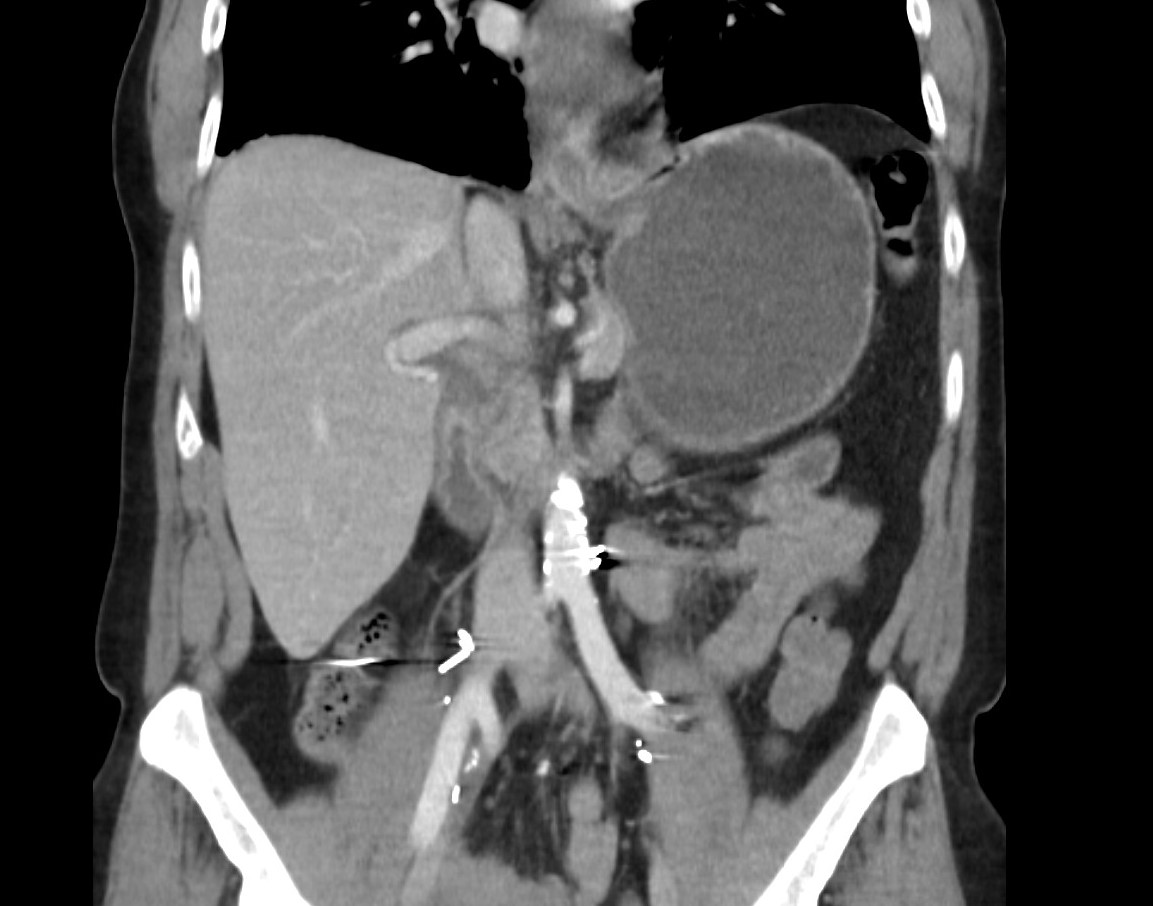
Figure: Figure 1. Radiologic imaging: Coronal abdomen computed tomography scan image showing extensive esophageal inflammation and thickening, indicative of severe esophagitis, which developed as a result of Durvalumab treatment in a patient with advanced urothelial cancer.
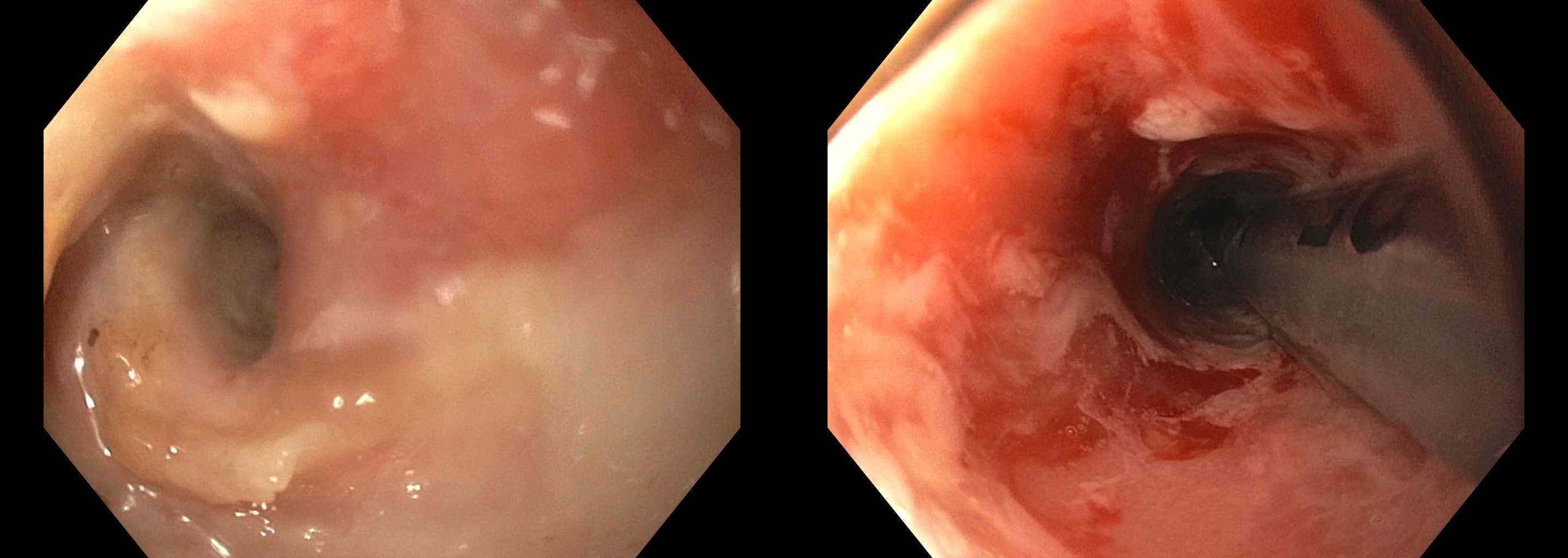
Figure: Figure 2. Endoscopic imaging. Endoscopic view of the esophagus showing severe inflammation, erythema, and erosions, consistent with severe esophagitis, observed 84 days after the initiation of Durvalumab therapy in a patient with advanced urothelial cancer.
Disclosures:
Kelly Vo indicated no relevant financial relationships.
Nathaniel Goss indicated no relevant financial relationships.
Michael Adams indicated no relevant financial relationships.
Patrick Young indicated no relevant financial relationships.
Kelly M. Vo, DO, Nathaniel Goss, MD, Michael Adams, DO, Patrick Young, MD, FACG. P5014 - Severe Esophagitis Induced by Durvalumab in the Treatment of Advanced Urothelial Cancer: A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Walter Reed National Military Medical Center, Bethesda, MD
Introduction: Durvalumab, an immune checkpoint inhibitor targeting PD-L1, shows promise in treating advanced urothelial cell cancer. Though generally well tolerated, it can lead to immune-related adverse events (irAEs). These have been observed in non-small cell lung cancer treatment but not, to date, in advanced urothelial cell bladder cancer. We present a case of severe esophagitis in a patient receiving durvalumab for advanced bladder cancer, underscoring the need for close monitoring.
Case Description/
Methods: A 58-year-old man with Stage IIIA (pT3aN0[0/39]M0) muscle-invasive bladder cancer (MIBC) with a predominant sarcomatoid urothelial variant and low-risk localized prostate cancer (pT2N0[0/39]) underwent open radical cystoprostatectomy, retroperitoneal lymph node dissection, and ileal conduit creation in August 2024. Three months later, he began neoadjuvant therapy with cisplatin, gemcitabine, and durvalumab, followed by adjuvant monotherapy with durvalumab. After two cycles of monotherapy, he developed severe dysphagia, nausea, and vomiting, 84 days after initiating chemotherapy. Hospital evaluation revealed stable vital signs and abdominal tenderness. A computed tomography scan showed distal esophageal thickening and gastric dilation (Figure 1). Esophagogastroduodenoscopy revealed severe esophagitis with mucosal damage (Figure 2). He was diagnosed with grade 3 ICI-induced esophagitis and treated with intravenous steroids, later transitioned to oral prednisone. His symptoms improved, and he was discharged on a steroid taper. Unfortunately, he died 25 days later; the cause of death remains unknown due to the absence of an autopsy.
Discussion: Durvalumab is FDA-approved for muscle-invasive Stage III urothelial cancer following cystectomy, with improved survival demonstrated in the NIAGARA trial. While gastrointestinal irAEs such as colitis are known, esophagitis is rare. In NSCLC, esophagitis occurred in 6.3% of patients post-chemoradiotherapy, typically during durvalumab treatment. The timing in this case aligns with previously reported patterns, and symptoms responded to glucocorticoids. Gemcitabine and cisplatin are not typically associated with esophagitis unless used with radiation. This is the first documented case of esophagitis related to durvalumab in urothelial cancer. Although the patient improved in hospital, the cause of his death post-discharge remains unclear but is likely unrelated to the irAE given his clinical improvement.

Figure: Figure 1. Radiologic imaging: Coronal abdomen computed tomography scan image showing extensive esophageal inflammation and thickening, indicative of severe esophagitis, which developed as a result of Durvalumab treatment in a patient with advanced urothelial cancer.

Figure: Figure 2. Endoscopic imaging. Endoscopic view of the esophagus showing severe inflammation, erythema, and erosions, consistent with severe esophagitis, observed 84 days after the initiation of Durvalumab therapy in a patient with advanced urothelial cancer.
Disclosures:
Kelly Vo indicated no relevant financial relationships.
Nathaniel Goss indicated no relevant financial relationships.
Michael Adams indicated no relevant financial relationships.
Patrick Young indicated no relevant financial relationships.
Kelly M. Vo, DO, Nathaniel Goss, MD, Michael Adams, DO, Patrick Young, MD, FACG. P5014 - Severe Esophagitis Induced by Durvalumab in the Treatment of Advanced Urothelial Cancer: A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
