Tuesday Poster Session
Category: Esophagus
P4977 - High-risk Tissue Systems Pathology Test (TSP-9) Results Enable Risk-aligned Management of Patients With Presumed Clinically Low-Risk Non-Dysplastic Barrett’s Esophagus
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- EH
Edward Horvath, MD
Gastro Health
Boynton Beach, FL
Presenting Author(s)
Award: ACG Presidential Poster Award
Edward Horvath, MD1, Ronen Arai, MD2, Seth Kirschner, DO2
1Gastro Health, Boynton Beach, FL; 2Gastro Health, Coral Springs, FL
Introduction: Current GI society guidelines recommend endoscopic surveillance for Non-dysplastic Barrett’s esophagus (NDBE) at 3–5-year intervals. However, a subset of patients with NDBE can progress to high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) within this timeframe; failure to identify these high-risk patients presents a challenge for reducing the incidence and mortality of EAC. The tissue systems pathology test (TissueCypher, TSP-9) has been validated to predict a patient’s individualized risk of progression to HGD/EAC within 5 years. These cases demonstrate how TSP-9 results guide personalized care for patients with NDBE.
Case Description/
Methods: Patient A is a 54 y/o Caucasian male with a 12-year history of stable Barrett’s esophagus (BE) described as a C3M4, NDBE lesion. At his last surveillance endoscopy, biopsies were sent for TSP-9 testing and revealed a high-risk score with a 5-year probability of progression of 43% (Figure 1A). This led to a risk-aligned recommendation for endoscopic eradication therapy (EET). At subsequent referral to an interventional endoscopist for EET, repeat biopsies at 6 months interval from TSP-9 testing demonstrated progression to Low Grade Dysplasia (LGD) as confirmed by two GI pathologists. Although the current plan for EET was temporarily interrupted due to other medical co-morbidities, the patient is slated for eventual EET rather than routine surveillance biopsies only.
Patient B is a 71 y/o Caucasian female with a short segment (C0M1), NDBE lesion. TSP-9 testing revealed a high-risk score corresponding to a 5-year probability of progression of 45% (Figure 1B). Risk-benefit discussions guided by the TSP-9 test led the patient to elect short-interval (1-yr) surveillance in lieu of EET. At 1-yr follow, there was no change in histological grade of BE, but the 2-yr follow-up demonstrated progression to LGD as confirmed by three GI pathologists. This progression preceded the 3–5-year surveillance interval recommended in current guidelines. Hence, the patient was reconsidered for EET discussion and decided to proceed with EET.
Discussion: These cases demonstrate that TSP-9 can provide individualized risk stratification for patients with NDBE, predicting progression of dysplasia earlier than guideline recommended surveillance interval time. The high-risk TSP-9 results enabled risk-aligned decision making for both patients that guided the decision for EET to prevent progression at an earlier, more treatable stage.
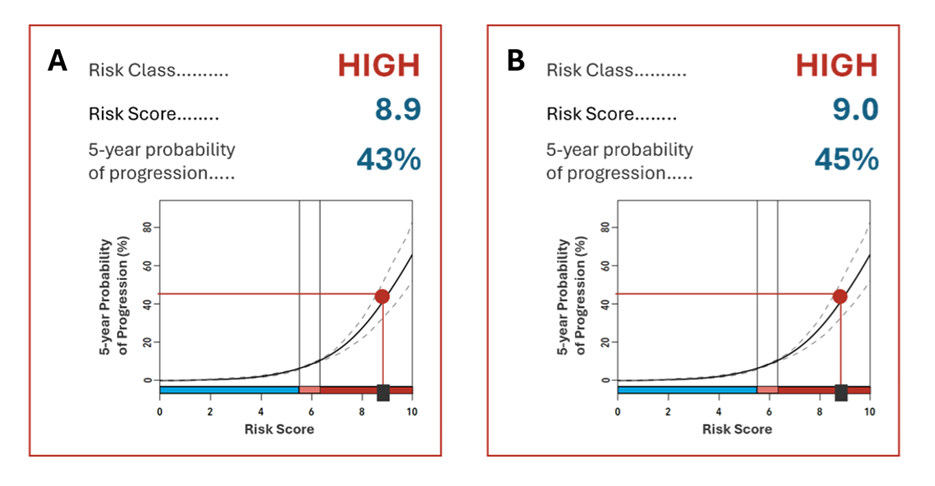
Figure: Figure 1. (A) Esophageal biopsies from Patient A were sent for TSP-9 testing and returned a high-risk score of 8.9 corresponding to a 43% probability of progression to HGD/EAC over 5 years. (B) TSP-9 testing for Patient B returned a high-risk score of 9.0 corresponding to a 45% probability of progression to HGD/EAC over 5 years.
Disclosures:
Edward Horvath: Castle Biosciences – Speakers Bureau.
Ronen Arai: Abbvie – Speakers Bureau. Castle Biosciences – Speakers Bureau. Celltrion – Speakers Bureau. Eli Lilly – Speakers Bureau. Johnson & Johnson – Speakers Bureau. Pfizer – Speakers Bureau. Phathom – Speakers Bureau.
Seth Kirschner indicated no relevant financial relationships.
Edward Horvath, MD1, Ronen Arai, MD2, Seth Kirschner, DO2. P4977 - High-risk Tissue Systems Pathology Test (TSP-9) Results Enable Risk-aligned Management of Patients With Presumed Clinically Low-Risk Non-Dysplastic Barrett’s Esophagus, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Edward Horvath, MD1, Ronen Arai, MD2, Seth Kirschner, DO2
1Gastro Health, Boynton Beach, FL; 2Gastro Health, Coral Springs, FL
Introduction: Current GI society guidelines recommend endoscopic surveillance for Non-dysplastic Barrett’s esophagus (NDBE) at 3–5-year intervals. However, a subset of patients with NDBE can progress to high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) within this timeframe; failure to identify these high-risk patients presents a challenge for reducing the incidence and mortality of EAC. The tissue systems pathology test (TissueCypher, TSP-9) has been validated to predict a patient’s individualized risk of progression to HGD/EAC within 5 years. These cases demonstrate how TSP-9 results guide personalized care for patients with NDBE.
Case Description/
Methods: Patient A is a 54 y/o Caucasian male with a 12-year history of stable Barrett’s esophagus (BE) described as a C3M4, NDBE lesion. At his last surveillance endoscopy, biopsies were sent for TSP-9 testing and revealed a high-risk score with a 5-year probability of progression of 43% (Figure 1A). This led to a risk-aligned recommendation for endoscopic eradication therapy (EET). At subsequent referral to an interventional endoscopist for EET, repeat biopsies at 6 months interval from TSP-9 testing demonstrated progression to Low Grade Dysplasia (LGD) as confirmed by two GI pathologists. Although the current plan for EET was temporarily interrupted due to other medical co-morbidities, the patient is slated for eventual EET rather than routine surveillance biopsies only.
Patient B is a 71 y/o Caucasian female with a short segment (C0M1), NDBE lesion. TSP-9 testing revealed a high-risk score corresponding to a 5-year probability of progression of 45% (Figure 1B). Risk-benefit discussions guided by the TSP-9 test led the patient to elect short-interval (1-yr) surveillance in lieu of EET. At 1-yr follow, there was no change in histological grade of BE, but the 2-yr follow-up demonstrated progression to LGD as confirmed by three GI pathologists. This progression preceded the 3–5-year surveillance interval recommended in current guidelines. Hence, the patient was reconsidered for EET discussion and decided to proceed with EET.
Discussion: These cases demonstrate that TSP-9 can provide individualized risk stratification for patients with NDBE, predicting progression of dysplasia earlier than guideline recommended surveillance interval time. The high-risk TSP-9 results enabled risk-aligned decision making for both patients that guided the decision for EET to prevent progression at an earlier, more treatable stage.

Figure: Figure 1. (A) Esophageal biopsies from Patient A were sent for TSP-9 testing and returned a high-risk score of 8.9 corresponding to a 43% probability of progression to HGD/EAC over 5 years. (B) TSP-9 testing for Patient B returned a high-risk score of 9.0 corresponding to a 45% probability of progression to HGD/EAC over 5 years.
Disclosures:
Edward Horvath: Castle Biosciences – Speakers Bureau.
Ronen Arai: Abbvie – Speakers Bureau. Castle Biosciences – Speakers Bureau. Celltrion – Speakers Bureau. Eli Lilly – Speakers Bureau. Johnson & Johnson – Speakers Bureau. Pfizer – Speakers Bureau. Phathom – Speakers Bureau.
Seth Kirschner indicated no relevant financial relationships.
Edward Horvath, MD1, Ronen Arai, MD2, Seth Kirschner, DO2. P4977 - High-risk Tissue Systems Pathology Test (TSP-9) Results Enable Risk-aligned Management of Patients With Presumed Clinically Low-Risk Non-Dysplastic Barrett’s Esophagus, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

