Tuesday Poster Session
Category: Esophagus
P4936 - Efficacy and Safety of Tislelizumab in Esophageal Squamous Cell Carcinoma: A Proportionality Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Allah Dad, MD (he/him/his)
Shiekh Zayed Medical College Rahim Yar Khan, Pakistan
Kot Addu, Punjab, Pakistan
Presenting Author(s)
Haris Mumtaz Malik, MBBS1, Arun Kumar. Maloth, MBBS2, Adnan Bhat, MD3, Muhammad Ansab, 4, Muhammad Ahsan Asif, MBBS5, Mariam Shahabi, MBBS6, Imran A. Reshi, MBBS7, Ayesha Arshad, MBBS6, Samiullah Shaikh, MD8, Allah Dad, MD9, Omar Al-Radideh, MD10, Waseem Nabi, MD11
1Rawalpindi Medical University, Rawalpindi, Punjab, Pakistan; 2Kakatiya Medical College, Warangal, India, Warangal, Telangana, India; 3University of Florida, Gainesville, FL; 4Services Institute of Medical Sciences, Lahore, Punjab, Pakistan; 5Jinnah Hospital Lahore, Lahore, Punjab, Pakistan; 6Dow Medical College, Karachi, Sindh, Pakistan; 7Sher i Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India; 8Liaquat University of Medical and Health Science, Hyderabad, Sindh, Pakistan; 9Shiekh Zayed Medical College Rahim Yar Khan, Pakistan, Kot Addu, Punjab, Pakistan; 10University of Florida College of Medicine, Gainesville, FL; 11Wyckoff Heights Medical Center, Brooklyn, NY
Introduction: Tislelizumab, a humanized anti-PD-1 monoclonal antibody, has shown promising antitumor activity in esophageal squamous cell carcinoma (ESCC), a malignancy with poor prognosis and limited therapeutic options. While individual studies report variable outcomes, a comprehensive meta-analysis quantifying its efficacy and safety is lacking. We aim to evaluate the pooled efficacy and safety outcomes of tislelizumab in ESCC using a proportionality meta-analysis.
Methods: A systematic literature search identified eligible prospective studies reporting survival and response outcomes in ESCC patients treated with tislelizumab (alone or in combination). Data were extracted for 1-year and 2-year overall survival (OS), 1-year and 2-year progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), pathological complete response (pCR), major pathological response (MPR), partial response rate (PRR), and adverse events (AEs), including grade ≥3 AEs. A random-effects proportional meta-analysis was performed using the DerSimonian-Laird method. Between-study heterogeneity was assessed by I² statistics.
Results: Nine studies involving 795 patients were included. The pooled 1-year overall survival (OS) was 75% (95% CI: 58–89%), and 2-year OS was 56% (95% CI: 36–74%). The 1-year progression-free survival (PFS) rate was 96% (95% CI: 93–99%) and 2-year PFS was 48% (95% CI: 26-71%). The objective response rate (ORR) reached 81% (95% CI: 51–99%), with a disease control rate (DCR) of 47% (95% CI: 34–59%). Pathological complete response (pCR) and major pathological response (MPR) were both estimated at 53%, while the partial response rate (PRR) was 36%. All-grade adverse events occurred in 23% of patients, and grade ≥3 adverse events in 58%. Overall, tislelizumab showed promising efficacy and manageable safety in ESCC.
Discussion: Tislelizumab demonstrates encouraging efficacy in ESCC with high 1-year PFS and OS rates, notable response rates (ORR, DCR, pCR), and manageable safety profile. The observed heterogeneity across studies underscores the need for further prospective randomized trials to optimize patient selection and combination strategies.
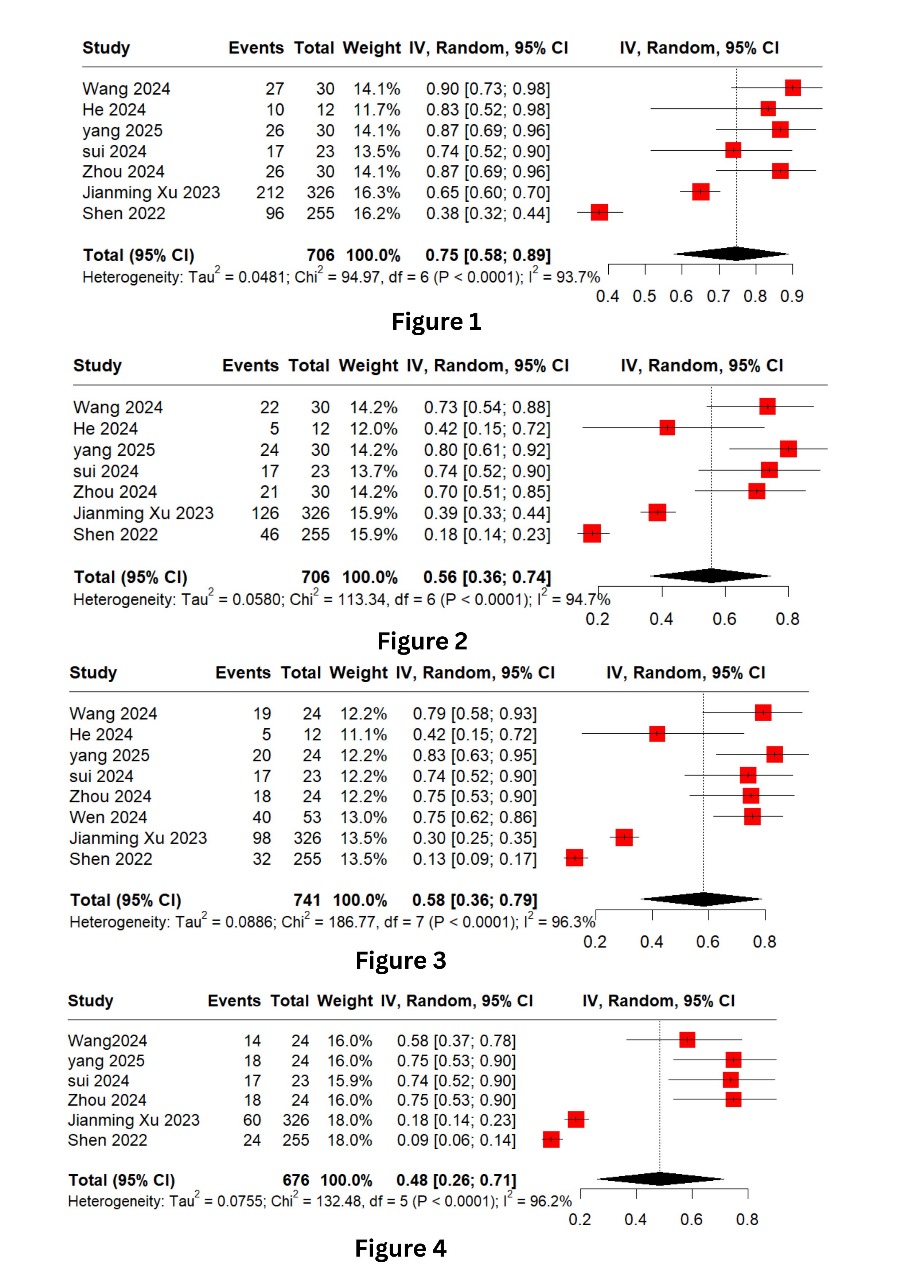
Figure: Title: Forest Plots
Figure 1: Overall Survival 1 year
Figure 2: Overall Survival 2 year
Figure 3: Progression Free Survival 1 year
Figure 4: Progression Free Survival 2 year
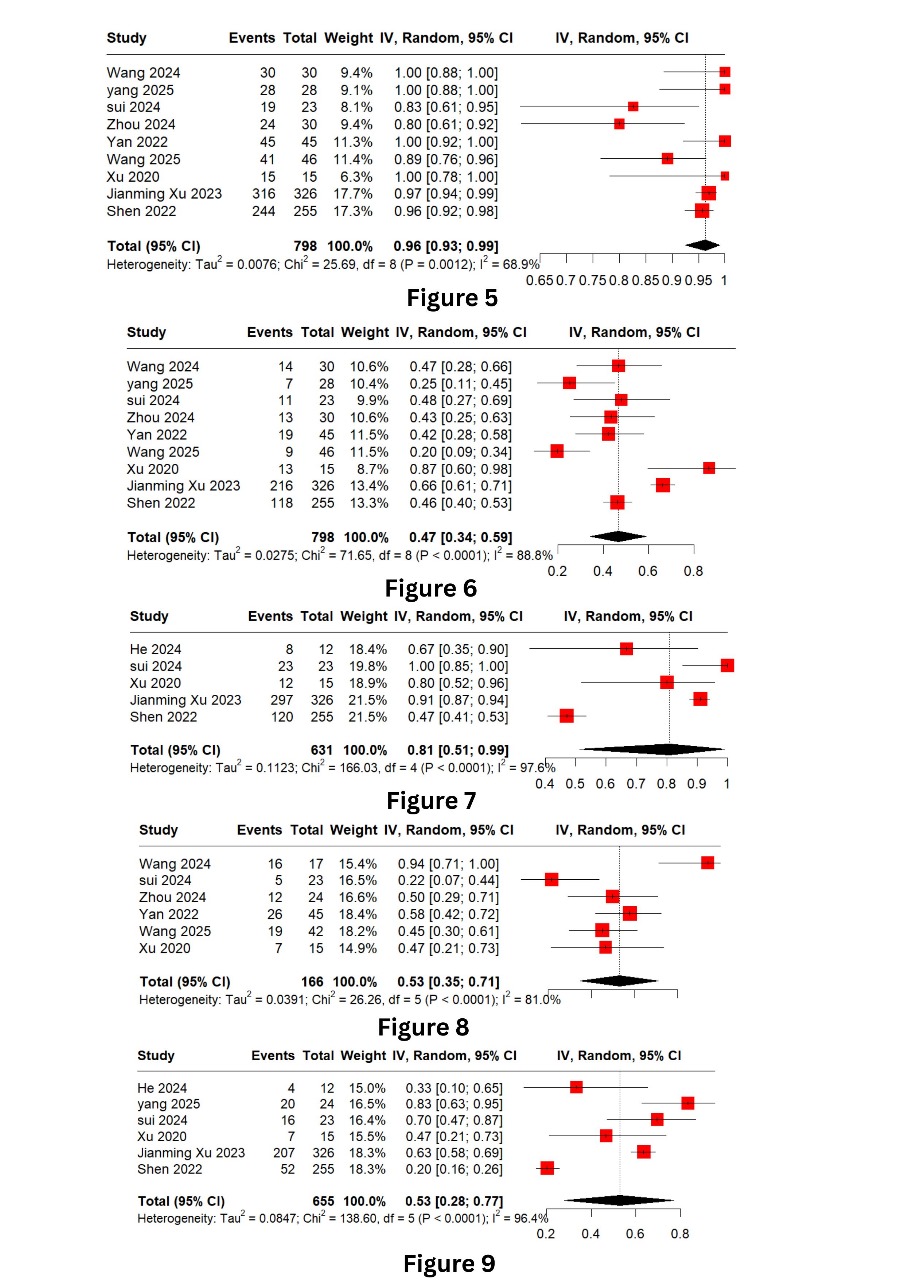
Figure: Title: Forest Plots
Figure 5: Adverse Events
Figure 6: Adverse Events (Grade 3 or >3)
Figure 7: Disease Control Rate
Figure 8: Major Pathological Response
Figure 9: Objective Response Rate
Disclosures:
Haris Mumtaz Malik indicated no relevant financial relationships.
Arun Maloth indicated no relevant financial relationships.
Adnan Bhat indicated no relevant financial relationships.
Muhammad Ansab indicated no relevant financial relationships.
Muhammad Ahsan Asif indicated no relevant financial relationships.
Mariam Shahabi indicated no relevant financial relationships.
Imran Reshi indicated no relevant financial relationships.
Ayesha Arshad indicated no relevant financial relationships.
Samiullah Shaikh indicated no relevant financial relationships.
Allah Dad indicated no relevant financial relationships.
Omar Al-Radideh indicated no relevant financial relationships.
Waseem Nabi indicated no relevant financial relationships.
Haris Mumtaz Malik, MBBS1, Arun Kumar. Maloth, MBBS2, Adnan Bhat, MD3, Muhammad Ansab, 4, Muhammad Ahsan Asif, MBBS5, Mariam Shahabi, MBBS6, Imran A. Reshi, MBBS7, Ayesha Arshad, MBBS6, Samiullah Shaikh, MD8, Allah Dad, MD9, Omar Al-Radideh, MD10, Waseem Nabi, MD11. P4936 - Efficacy and Safety of Tislelizumab in Esophageal Squamous Cell Carcinoma: A Proportionality Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Rawalpindi Medical University, Rawalpindi, Punjab, Pakistan; 2Kakatiya Medical College, Warangal, India, Warangal, Telangana, India; 3University of Florida, Gainesville, FL; 4Services Institute of Medical Sciences, Lahore, Punjab, Pakistan; 5Jinnah Hospital Lahore, Lahore, Punjab, Pakistan; 6Dow Medical College, Karachi, Sindh, Pakistan; 7Sher i Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India; 8Liaquat University of Medical and Health Science, Hyderabad, Sindh, Pakistan; 9Shiekh Zayed Medical College Rahim Yar Khan, Pakistan, Kot Addu, Punjab, Pakistan; 10University of Florida College of Medicine, Gainesville, FL; 11Wyckoff Heights Medical Center, Brooklyn, NY
Introduction: Tislelizumab, a humanized anti-PD-1 monoclonal antibody, has shown promising antitumor activity in esophageal squamous cell carcinoma (ESCC), a malignancy with poor prognosis and limited therapeutic options. While individual studies report variable outcomes, a comprehensive meta-analysis quantifying its efficacy and safety is lacking. We aim to evaluate the pooled efficacy and safety outcomes of tislelizumab in ESCC using a proportionality meta-analysis.
Methods: A systematic literature search identified eligible prospective studies reporting survival and response outcomes in ESCC patients treated with tislelizumab (alone or in combination). Data were extracted for 1-year and 2-year overall survival (OS), 1-year and 2-year progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), pathological complete response (pCR), major pathological response (MPR), partial response rate (PRR), and adverse events (AEs), including grade ≥3 AEs. A random-effects proportional meta-analysis was performed using the DerSimonian-Laird method. Between-study heterogeneity was assessed by I² statistics.
Results: Nine studies involving 795 patients were included. The pooled 1-year overall survival (OS) was 75% (95% CI: 58–89%), and 2-year OS was 56% (95% CI: 36–74%). The 1-year progression-free survival (PFS) rate was 96% (95% CI: 93–99%) and 2-year PFS was 48% (95% CI: 26-71%). The objective response rate (ORR) reached 81% (95% CI: 51–99%), with a disease control rate (DCR) of 47% (95% CI: 34–59%). Pathological complete response (pCR) and major pathological response (MPR) were both estimated at 53%, while the partial response rate (PRR) was 36%. All-grade adverse events occurred in 23% of patients, and grade ≥3 adverse events in 58%. Overall, tislelizumab showed promising efficacy and manageable safety in ESCC.
Discussion: Tislelizumab demonstrates encouraging efficacy in ESCC with high 1-year PFS and OS rates, notable response rates (ORR, DCR, pCR), and manageable safety profile. The observed heterogeneity across studies underscores the need for further prospective randomized trials to optimize patient selection and combination strategies.

Figure: Title: Forest Plots
Figure 1: Overall Survival 1 year
Figure 2: Overall Survival 2 year
Figure 3: Progression Free Survival 1 year
Figure 4: Progression Free Survival 2 year

Figure: Title: Forest Plots
Figure 5: Adverse Events
Figure 6: Adverse Events (Grade 3 or >3)
Figure 7: Disease Control Rate
Figure 8: Major Pathological Response
Figure 9: Objective Response Rate
Disclosures:
Haris Mumtaz Malik indicated no relevant financial relationships.
Arun Maloth indicated no relevant financial relationships.
Adnan Bhat indicated no relevant financial relationships.
Muhammad Ansab indicated no relevant financial relationships.
Muhammad Ahsan Asif indicated no relevant financial relationships.
Mariam Shahabi indicated no relevant financial relationships.
Imran Reshi indicated no relevant financial relationships.
Ayesha Arshad indicated no relevant financial relationships.
Samiullah Shaikh indicated no relevant financial relationships.
Allah Dad indicated no relevant financial relationships.
Omar Al-Radideh indicated no relevant financial relationships.
Waseem Nabi indicated no relevant financial relationships.
Haris Mumtaz Malik, MBBS1, Arun Kumar. Maloth, MBBS2, Adnan Bhat, MD3, Muhammad Ansab, 4, Muhammad Ahsan Asif, MBBS5, Mariam Shahabi, MBBS6, Imran A. Reshi, MBBS7, Ayesha Arshad, MBBS6, Samiullah Shaikh, MD8, Allah Dad, MD9, Omar Al-Radideh, MD10, Waseem Nabi, MD11. P4936 - Efficacy and Safety of Tislelizumab in Esophageal Squamous Cell Carcinoma: A Proportionality Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
