Tuesday Poster Session
Category: Esophagus
P4935 - Real-World Effectiveness of Dupilumab in Patients With Eosinophilic Esophagitis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Carl Olson, BA
University of Minnesota Medical School
Minneapolis, MN
Presenting Author(s)
Carl Olson, BA1, Jameel Alp, MD2, Joshua Sloan, DO2
1University of Minnesota Medical School, Minneapolis, MN; 2University of Minnesota, Minneapolis, MN
Introduction: Eosinophilic esophagitis (EoE) is a chronic inflammatory disease defined by eosinophils within the esophageal mucosa, typically presenting with dysphagia and often progressing to fibrostenotic changes. Dupilumab, an IL-4/IL-13 inhibitor, was approved for the treatment of EoE in 2022 based on a phase 3 clinical trial[1]. Real-world data remains limited, especially outside of the clinical trial and quaternary referral centers. This study assessed dupilumab’s effectiveness in a tertiary care setting uninvolved in prior trials.
Methods: We conducted a retrospective cohort study of adults (≥18 years) with confirmed EoE on biopsy (≥15 eos/hpf) who initiated dupilumab therapy between 1/2022 and 10/2024. Clinical outcomes were assessed at baseline and at follow-up visits after starting therapy. Measures included symptom burden (BEDQ, Eckardt), endoscopic severity (EREFS), histologic activity (peak eos/hpf), and adverse events (AE). The primary endpoint was histologic remission (< 15 eos/hpf). Pre/post-dupilumab changes were tested for significance via the Wilcoxon signed-rank test.
Results: 44 patients were included (see Table 1). From baseline to follow-up 1 (median 20.5 weeks), paired BEDQ scores decreased by a median of 4.5 (n=10; p=0.0076) and Eckardt scores by 1.0 (n=10; p=0.0160). EREFS scores declined by a median of 2.0 from baseline to follow-up 1 (n=29; p< 0.0001), with sustained improvement at follow-up 2 (Δ=2.0, n=10; p=0.0169). Peak eosinophil burden fell by 47.5 (n=26; p< 0.0001) at a follow up 1, which was sustained at follow follow-up 2 (Δ=37.0, n=11; p=0.0069), with no significant change between follow-up 1 and 2 (n=11, p=0.31). Histologic remission was achieved in 76.9% at follow-up 1 and 72.7% at follow-up 2. Paired comparisons are detailed in Table 2. 15.9% of patients reported AEs and 9.1% discontinued therapy.
Discussion: In this cohort from a general practice setting, dupilumab was associated with durable histologic and endoscopic improvements and early symptom relief. These findings are consistent with the clinical trial and recent observational data, supporting dupilumab’s effectiveness and generalizability in real-world practice. AEs were less frequent in our study (15.9%) when compared to the clinical trial (60-86%)[1]. These findings add to growing evidence that dupilumab is effective in the real-world environment.
[1] Dellon ES, Rothenberg ME, Collins MH, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022;387(25):2317-2330.
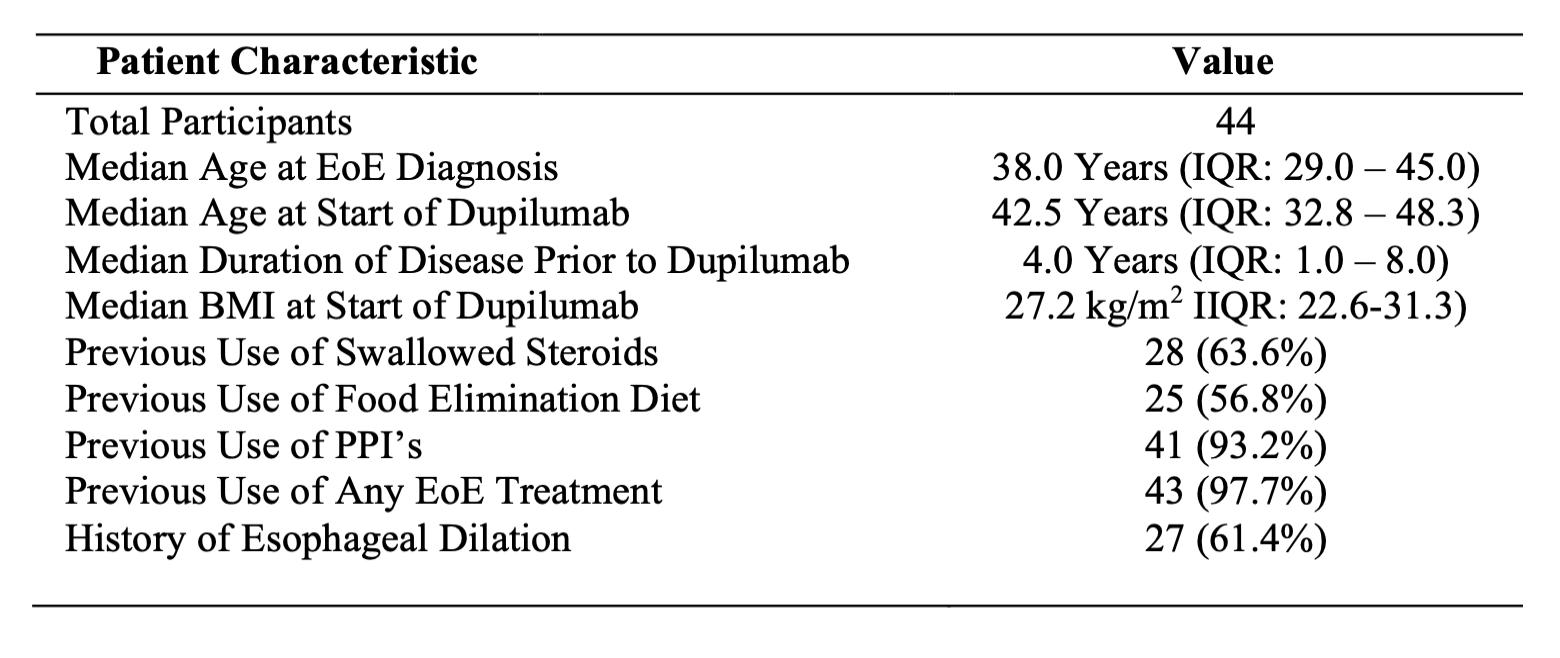
Figure: Table 1. Baseline demographic and clinical characteristics of the study cohort
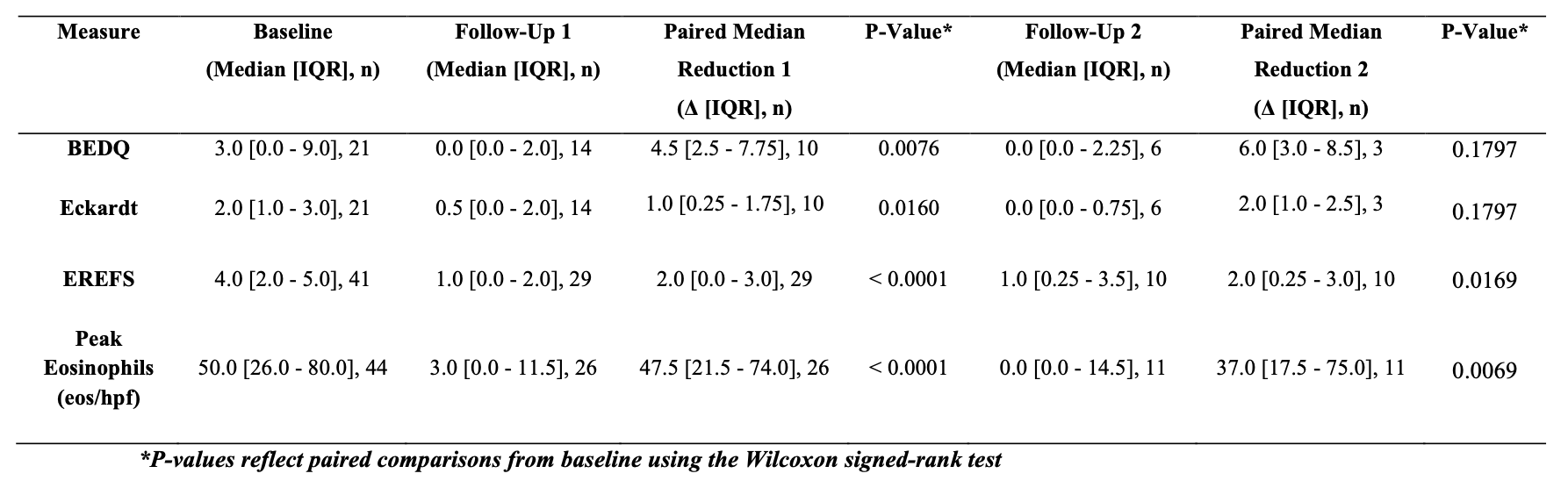
Figure: Table 2. Median Symptom, Endoscopic, and Histologic Outcomes from Baseline to Follow-Up on Dupilumab
Disclosures:
Carl Olson indicated no relevant financial relationships.
Jameel Alp indicated no relevant financial relationships.
Joshua Sloan: Phathom Pharmaceuticals – Speakers Bureau. Sanofi-Regeneron – Advisory Committee/Board Member, Speakers Bureau. Takeda Pharmaceuticals – Advisory Committee/Board Member.
Carl Olson, BA1, Jameel Alp, MD2, Joshua Sloan, DO2. P4935 - Real-World Effectiveness of Dupilumab in Patients With Eosinophilic Esophagitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Minnesota Medical School, Minneapolis, MN; 2University of Minnesota, Minneapolis, MN
Introduction: Eosinophilic esophagitis (EoE) is a chronic inflammatory disease defined by eosinophils within the esophageal mucosa, typically presenting with dysphagia and often progressing to fibrostenotic changes. Dupilumab, an IL-4/IL-13 inhibitor, was approved for the treatment of EoE in 2022 based on a phase 3 clinical trial[1]. Real-world data remains limited, especially outside of the clinical trial and quaternary referral centers. This study assessed dupilumab’s effectiveness in a tertiary care setting uninvolved in prior trials.
Methods: We conducted a retrospective cohort study of adults (≥18 years) with confirmed EoE on biopsy (≥15 eos/hpf) who initiated dupilumab therapy between 1/2022 and 10/2024. Clinical outcomes were assessed at baseline and at follow-up visits after starting therapy. Measures included symptom burden (BEDQ, Eckardt), endoscopic severity (EREFS), histologic activity (peak eos/hpf), and adverse events (AE). The primary endpoint was histologic remission (< 15 eos/hpf). Pre/post-dupilumab changes were tested for significance via the Wilcoxon signed-rank test.
Results: 44 patients were included (see Table 1). From baseline to follow-up 1 (median 20.5 weeks), paired BEDQ scores decreased by a median of 4.5 (n=10; p=0.0076) and Eckardt scores by 1.0 (n=10; p=0.0160). EREFS scores declined by a median of 2.0 from baseline to follow-up 1 (n=29; p< 0.0001), with sustained improvement at follow-up 2 (Δ=2.0, n=10; p=0.0169). Peak eosinophil burden fell by 47.5 (n=26; p< 0.0001) at a follow up 1, which was sustained at follow follow-up 2 (Δ=37.0, n=11; p=0.0069), with no significant change between follow-up 1 and 2 (n=11, p=0.31). Histologic remission was achieved in 76.9% at follow-up 1 and 72.7% at follow-up 2. Paired comparisons are detailed in Table 2. 15.9% of patients reported AEs and 9.1% discontinued therapy.
Discussion: In this cohort from a general practice setting, dupilumab was associated with durable histologic and endoscopic improvements and early symptom relief. These findings are consistent with the clinical trial and recent observational data, supporting dupilumab’s effectiveness and generalizability in real-world practice. AEs were less frequent in our study (15.9%) when compared to the clinical trial (60-86%)[1]. These findings add to growing evidence that dupilumab is effective in the real-world environment.
[1] Dellon ES, Rothenberg ME, Collins MH, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022;387(25):2317-2330.

Figure: Table 1. Baseline demographic and clinical characteristics of the study cohort

Figure: Table 2. Median Symptom, Endoscopic, and Histologic Outcomes from Baseline to Follow-Up on Dupilumab
Disclosures:
Carl Olson indicated no relevant financial relationships.
Jameel Alp indicated no relevant financial relationships.
Joshua Sloan: Phathom Pharmaceuticals – Speakers Bureau. Sanofi-Regeneron – Advisory Committee/Board Member, Speakers Bureau. Takeda Pharmaceuticals – Advisory Committee/Board Member.
Carl Olson, BA1, Jameel Alp, MD2, Joshua Sloan, DO2. P4935 - Real-World Effectiveness of Dupilumab in Patients With Eosinophilic Esophagitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
