Tuesday Poster Session
Category: Esophagus
P4901 - Real-World Experience With Non-Endoscopic Screening for Barrett’s Esophagus (BE): Strong Guideline Adherence With Highest Positivity in Patients Meeting American College of Gastroenterology (ACG) BE Screening Criteria
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Natalie J. Wilson, MD (she/her/hers)
University of Minnesota
Chapel Hill, NC
Presenting Author(s)
Award: ACG Presidential Poster Award
Natalie J. Wilson, MD1, Victoria T. Lee, MD2, Suman Verma, MD, PhD2, Lishan Aklog, MD2, Nicholas J. Shaheen, MD, MPH, MACG3
1University of Minnesota, Minneapolis, MN; 2LucidDx, New York, NY; 3University of North Carolina at Chapel Hill, Chapel Hill, NC
Introduction: Non-endoscopic biomarker screening for Barrett’s esophagus (BE) is commercially available in the U.S. However, it’s real-world use outside of research settings is not described. The American College of Gastroenterology (ACG) recommends these tests as an alternative to endoscopy in patients with gastroesophageal reflux (GERD) and at least 3 of 6 additional risk factors. The American Gastroenterological Association (AGA) also recommends screening in patients with 3 risk factors, but includes chronic GERD as just one of 7 risk factors. This study aimed to evaluate the real-world use of these tests, focusing on 1) adherence to societal guidelines for patient selection, 2) the impact of societal screening criteria on the number of patients screened, and 3) the impact of these criteria on the likelihood of a positive test.
Methods: This retrospective study analyzed data from a commercial database of EsoCheck® (EC, Lucid Diagnostics, NY, NY), a swallowable balloon capsule device, followed by EsoGuard® (EG, Lucid), a methylated biomarker test (mVIM and mCCNA1), performed in an office or health fair setting. Data from the laboratory’s clinical database were analyzed in an IRB-approved study.
Results: Between January 2023 and June 2024, 11,355 patients underwent testing, with a binary result reported in 10,566 (93.4%). Demographics, BE risk factors, and adherence to ACG/AGA BE screening criteria are shown in Table 1. Overall, 83.6% of patients met at least one society’s criteria. Almost twice as many patients met AGA criteria as ACG criteria. Nearly half of the AGA cohort did not have symptomatic GERD.
Overall and cohort EG positivity rates are presented in Table 2. The overall positivity rate was 16.6%. The AGA cohort had a lower positivity rate than the ACG cohort (p < 0.01). The non-GERD AGA cohort had a lower positivity rate than either the ACG cohort or full AGA cohort (p < 0.0001). The cohort not meeting societal criteria had the lowest positivity rate.
Discussion: The vast majority of patients in this real-world cohort undergoing non-endoscopic biomarker screening for BE met at least one professional society’s criteria, indicating strong guideline adherence. Use of the less stringent AGA criteria doubled the number of patients screened while use of the more stringent ACG criteria resulted in a higher yield of the test, especially compared to a non-GERD AGA cohort. Over 3/4ths of patients had a negative EG test and required no further testing.
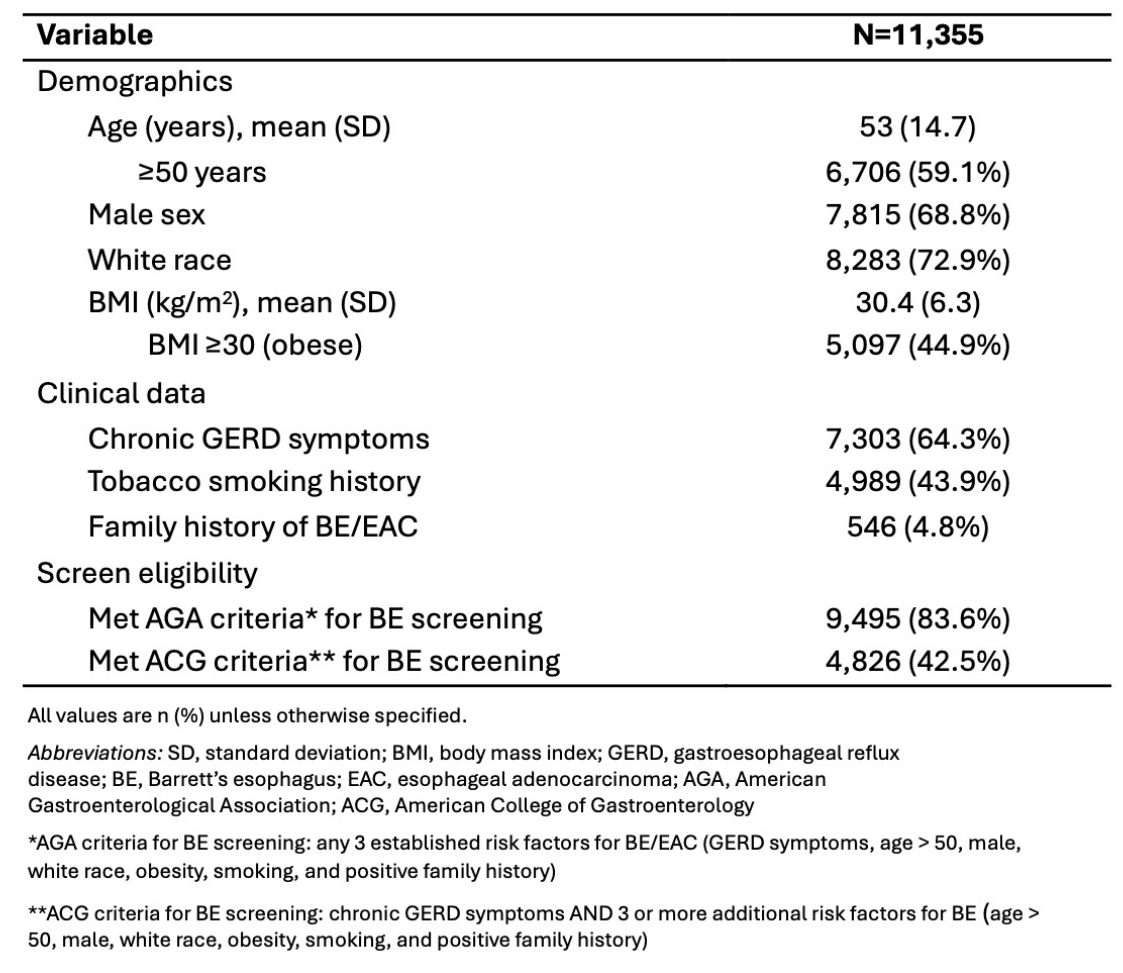
Figure: Table 1. Characteristics of screened subjects and risk factors for Barrett’s esophagus
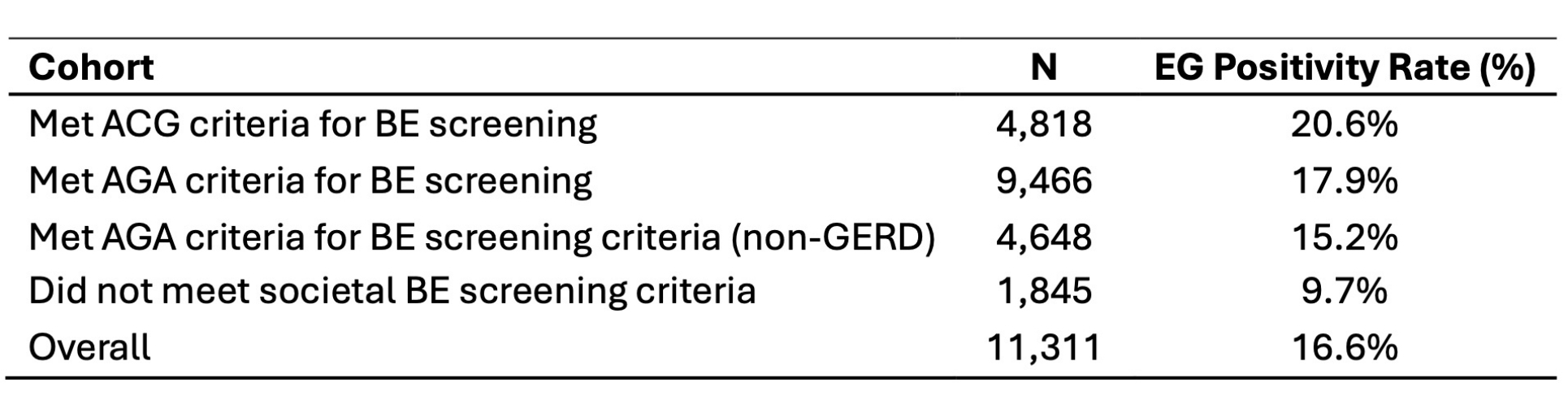
Figure: Table 2. EsoGuard Positivity Rate by Societal Screening Criteria Used
Disclosures:
Natalie Wilson indicated no relevant financial relationships.
Victoria Lee: Lucid Diagnostics Inc. – Employee, Stock Options.
Suman Verma: Lucid Diagnostics – Employee.
Lishan Aklog: Lucid Diagnostics – Employee, Intellectual Property/Patents, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Nicholas Shaheen: Aqua – Grant/Research Support. CDx – Grant/Research Support. Cook Medical – Consultant. GIE Medical – Grant/Research Support. Interpace Diagnostics – Grant/Research Support. Lucid Diagnostics – Grant/Research Support. Medtronic – Grant/Research Support. Pentax – Grant/Research Support. Steris – Grant/Research Support.
Natalie J. Wilson, MD1, Victoria T. Lee, MD2, Suman Verma, MD, PhD2, Lishan Aklog, MD2, Nicholas J. Shaheen, MD, MPH, MACG3. P4901 - Real-World Experience With Non-Endoscopic Screening for Barrett’s Esophagus (BE): Strong Guideline Adherence With Highest Positivity in Patients Meeting American College of Gastroenterology (ACG) BE Screening Criteria, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Natalie J. Wilson, MD1, Victoria T. Lee, MD2, Suman Verma, MD, PhD2, Lishan Aklog, MD2, Nicholas J. Shaheen, MD, MPH, MACG3
1University of Minnesota, Minneapolis, MN; 2LucidDx, New York, NY; 3University of North Carolina at Chapel Hill, Chapel Hill, NC
Introduction: Non-endoscopic biomarker screening for Barrett’s esophagus (BE) is commercially available in the U.S. However, it’s real-world use outside of research settings is not described. The American College of Gastroenterology (ACG) recommends these tests as an alternative to endoscopy in patients with gastroesophageal reflux (GERD) and at least 3 of 6 additional risk factors. The American Gastroenterological Association (AGA) also recommends screening in patients with 3 risk factors, but includes chronic GERD as just one of 7 risk factors. This study aimed to evaluate the real-world use of these tests, focusing on 1) adherence to societal guidelines for patient selection, 2) the impact of societal screening criteria on the number of patients screened, and 3) the impact of these criteria on the likelihood of a positive test.
Methods: This retrospective study analyzed data from a commercial database of EsoCheck® (EC, Lucid Diagnostics, NY, NY), a swallowable balloon capsule device, followed by EsoGuard® (EG, Lucid), a methylated biomarker test (mVIM and mCCNA1), performed in an office or health fair setting. Data from the laboratory’s clinical database were analyzed in an IRB-approved study.
Results: Between January 2023 and June 2024, 11,355 patients underwent testing, with a binary result reported in 10,566 (93.4%). Demographics, BE risk factors, and adherence to ACG/AGA BE screening criteria are shown in Table 1. Overall, 83.6% of patients met at least one society’s criteria. Almost twice as many patients met AGA criteria as ACG criteria. Nearly half of the AGA cohort did not have symptomatic GERD.
Overall and cohort EG positivity rates are presented in Table 2. The overall positivity rate was 16.6%. The AGA cohort had a lower positivity rate than the ACG cohort (p < 0.01). The non-GERD AGA cohort had a lower positivity rate than either the ACG cohort or full AGA cohort (p < 0.0001). The cohort not meeting societal criteria had the lowest positivity rate.
Discussion: The vast majority of patients in this real-world cohort undergoing non-endoscopic biomarker screening for BE met at least one professional society’s criteria, indicating strong guideline adherence. Use of the less stringent AGA criteria doubled the number of patients screened while use of the more stringent ACG criteria resulted in a higher yield of the test, especially compared to a non-GERD AGA cohort. Over 3/4ths of patients had a negative EG test and required no further testing.

Figure: Table 1. Characteristics of screened subjects and risk factors for Barrett’s esophagus

Figure: Table 2. EsoGuard Positivity Rate by Societal Screening Criteria Used
Disclosures:
Natalie Wilson indicated no relevant financial relationships.
Victoria Lee: Lucid Diagnostics Inc. – Employee, Stock Options.
Suman Verma: Lucid Diagnostics – Employee.
Lishan Aklog: Lucid Diagnostics – Employee, Intellectual Property/Patents, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Nicholas Shaheen: Aqua – Grant/Research Support. CDx – Grant/Research Support. Cook Medical – Consultant. GIE Medical – Grant/Research Support. Interpace Diagnostics – Grant/Research Support. Lucid Diagnostics – Grant/Research Support. Medtronic – Grant/Research Support. Pentax – Grant/Research Support. Steris – Grant/Research Support.
Natalie J. Wilson, MD1, Victoria T. Lee, MD2, Suman Verma, MD, PhD2, Lishan Aklog, MD2, Nicholas J. Shaheen, MD, MPH, MACG3. P4901 - Real-World Experience With Non-Endoscopic Screening for Barrett’s Esophagus (BE): Strong Guideline Adherence With Highest Positivity in Patients Meeting American College of Gastroenterology (ACG) BE Screening Criteria, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

