Tuesday Poster Session
Category: Diet, Nutrition, and Obesity
P4861 - Successful Management of Weight Regain Following Roux-en-Y Gastric Bypass Using the NXT Overstitch Suturing Device
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Razan Aburumman, MD
Henry Ford Health
Detroit, MI
Presenting Author(s)
Razan Aburumman, MD1, Barham Abu Dayyeh, MD, MPH2
1Henry Ford Health, Detroit, MI; 2Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: Roux-en-Y gastric bypass (RYGB) is a widely used bariatric procedure for weight loss and managing obesity-related conditions. Despite its effectiveness, long-term weight regain is a common issue, with over 25% of patients regaining significant weight within ten years. The diameter of the gastrojejunal anastomosis (GJA) is a key factor in this weight regain. Transoral outlet reduction (TORe) has been developed to address this, and newer devices like the NXT overstitch suturing device offer advanced features for this procedure.
Case Description/
Methods: A 50-year-old woman who underwent laparoscopic RYGB surgery at 37 years old in 2010. Initially weighing 262 pounds with a BMI of 42.7, she suffered from hypertension, obstructive sleep apnea, and GERD. She reached a nadir weight of 115 pounds and a BMI of 18.6. Her weight gradually increased, reaching a maximum weight of 253 lbs. 13 years later, so she was scheduled for a TORe procedure.
The gastroesophageal flap valve was classified as Hill Grade IV, and a 4 cm gastric pouch, half of which was herniated into the thorax through a hiatal hernia, was noted. The gastrojejunal anastomosis was dilated to 30 mm. APC was used to ablate mucosa around the anastomosis, and the overstitch NXT suturing device was used to place 8 stitches into the tissue ridge of the anastomosis. The suture was tightened and cinched, and another suture was placed in a U-shaped configuration to reduce the distal pouch into a tubular-shaped outlet. A successful zipper-like outlet closure was achieved and the outlet was estimated to be approximately 8mm. The patient was discharged the same day and successfully lost 35 lbs. within the first two months post-procedure.
Discussion: We discussed the application of the new NXT Overstitch endoscopic suturing device for performing an outlet revision case of weight regain following RYGB, complicated by a hiatal hernia. This device proved particularly valuable due to several advanced features. Its single-channel endoscope system provides a clear, unobstructed view and eliminates the need for double-lumen scopes. Additionally, the device's clutch system enables the tip to articulate at sharper angles, enhancing its ability to reach and maneuver within various anatomical sites and orientations. Lastly, the streamlined helical retractor operates in the same plane as the suturing arm, allowing for effective full-thickness tissue acquisition.
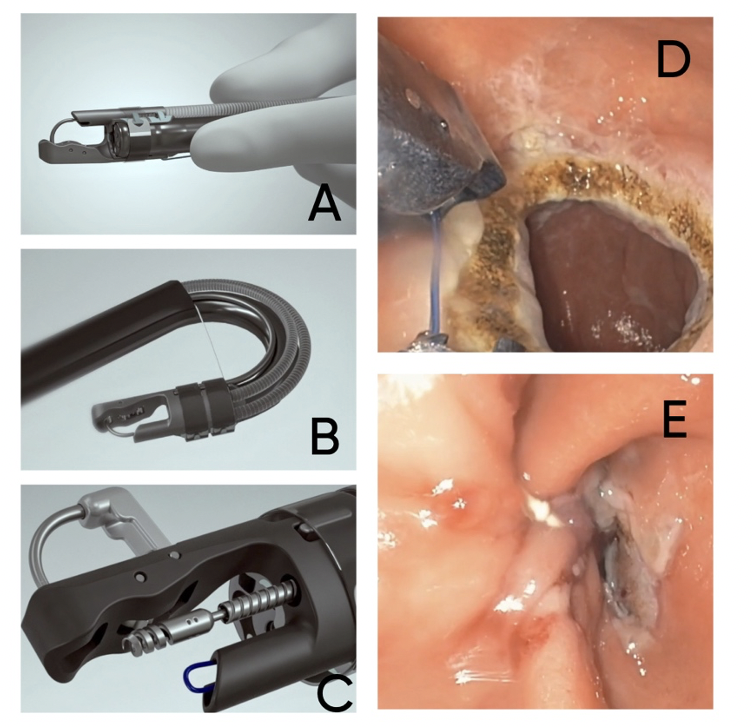
Figure: Figure 1: A) An image demonstrating the single-channel scope system, B) An image showing how the device can retroflex allowing suturing in different angles, C) An image showing the helical retractor, D) The gastric outlet before TORe and after APC, E) The gastric outlet after suturing
Disclosures:
Razan Aburumman indicated no relevant financial relationships.
Barham Abu Dayyeh: Boston Scientific, Medtronic, Apollo Endosurgery, and Olympus – Consultant. Boston Scientific, Medtronic, Apollo Endosurgery, and USGI Medical – Grant/Research Support. Endogenex technology licensed by Mayo Clinic, with institutional equity and royalty through Mayo Clinic's invention policy. – coinventor.
Razan Aburumman, MD1, Barham Abu Dayyeh, MD, MPH2. P4861 - Successful Management of Weight Regain Following Roux-en-Y Gastric Bypass Using the NXT Overstitch Suturing Device, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Henry Ford Health, Detroit, MI; 2Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: Roux-en-Y gastric bypass (RYGB) is a widely used bariatric procedure for weight loss and managing obesity-related conditions. Despite its effectiveness, long-term weight regain is a common issue, with over 25% of patients regaining significant weight within ten years. The diameter of the gastrojejunal anastomosis (GJA) is a key factor in this weight regain. Transoral outlet reduction (TORe) has been developed to address this, and newer devices like the NXT overstitch suturing device offer advanced features for this procedure.
Case Description/
Methods: A 50-year-old woman who underwent laparoscopic RYGB surgery at 37 years old in 2010. Initially weighing 262 pounds with a BMI of 42.7, she suffered from hypertension, obstructive sleep apnea, and GERD. She reached a nadir weight of 115 pounds and a BMI of 18.6. Her weight gradually increased, reaching a maximum weight of 253 lbs. 13 years later, so she was scheduled for a TORe procedure.
The gastroesophageal flap valve was classified as Hill Grade IV, and a 4 cm gastric pouch, half of which was herniated into the thorax through a hiatal hernia, was noted. The gastrojejunal anastomosis was dilated to 30 mm. APC was used to ablate mucosa around the anastomosis, and the overstitch NXT suturing device was used to place 8 stitches into the tissue ridge of the anastomosis. The suture was tightened and cinched, and another suture was placed in a U-shaped configuration to reduce the distal pouch into a tubular-shaped outlet. A successful zipper-like outlet closure was achieved and the outlet was estimated to be approximately 8mm. The patient was discharged the same day and successfully lost 35 lbs. within the first two months post-procedure.
Discussion: We discussed the application of the new NXT Overstitch endoscopic suturing device for performing an outlet revision case of weight regain following RYGB, complicated by a hiatal hernia. This device proved particularly valuable due to several advanced features. Its single-channel endoscope system provides a clear, unobstructed view and eliminates the need for double-lumen scopes. Additionally, the device's clutch system enables the tip to articulate at sharper angles, enhancing its ability to reach and maneuver within various anatomical sites and orientations. Lastly, the streamlined helical retractor operates in the same plane as the suturing arm, allowing for effective full-thickness tissue acquisition.

Figure: Figure 1: A) An image demonstrating the single-channel scope system, B) An image showing how the device can retroflex allowing suturing in different angles, C) An image showing the helical retractor, D) The gastric outlet before TORe and after APC, E) The gastric outlet after suturing
Disclosures:
Razan Aburumman indicated no relevant financial relationships.
Barham Abu Dayyeh: Boston Scientific, Medtronic, Apollo Endosurgery, and Olympus – Consultant. Boston Scientific, Medtronic, Apollo Endosurgery, and USGI Medical – Grant/Research Support. Endogenex technology licensed by Mayo Clinic, with institutional equity and royalty through Mayo Clinic's invention policy. – coinventor.
Razan Aburumman, MD1, Barham Abu Dayyeh, MD, MPH2. P4861 - Successful Management of Weight Regain Following Roux-en-Y Gastric Bypass Using the NXT Overstitch Suturing Device, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
