Tuesday Poster Session
Category: Diet, Nutrition, and Obesity
P4856 - Ischemic Colitis Following Tirzepatide Therapy: An Emerging Clinical Observation
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Alexa Plato, MD
Yale New Haven Health, Bridgeport Hospital
Bridgeport, CT
Presenting Author(s)
Alexa Plato, MD1, George M. Hanna, MD1, Raquel Rozner, MD2
1Yale New Haven Health, Bridgeport Hospital, Bridgeport, CT; 2Yale School of Medicine, Gastroenterology Associates, PC, Northeast Medical Group/Yale New Haven Health, Stratford, CT
Introduction: Tirzepatide is a novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist approved for the treatment of type 2 diabetes mellitus and obesity. While its efficacy in glycemic control and weight loss is well established, its safety profile continues to evolve. Gastrointestinal side effects such as nausea, vomiting and diarrhea are common; however, serious adverse events involving the gastrointestinal tract, including ischemic colitis, have not been widely reported. Here we present a case of ischemic colitis temporally associated with the initiation of tirzepatide.
Case Description/
Methods: A 45-year-old female with a history of anxiety, depression and class II obesity presented to the emergency department with two days of nausea and painful hematochezia. She reported passing multiple blood clots but no stool. Her symptoms began 7 days after initiating a 5 day course of cephalexin for impetigo. She denied fevers, chills or prior endoscopic evaluation. There were no recent changes to her medications which included fluoxetine, lithium and lorazepam. Tirzepatide was initiated 8 months prior to presentation, with her last dose administered 5 days prior to presentation. The patient denied NSAID use, alcohol, tobacco or drug use. Laboratory results were notable for a C-reactive protein of 83.5 mg/L and white blood cell count of 11.6 x10⁹/L. CT scan demonstrated nonspecific colitis involving the descending colon (Figure 1). Based on the clinical presentation and imaging, ischemic colitis was suspected. Tirzepatide was held and she was treated with 7 days of antibiotics. The patient reported complete resolution of symptoms at her 1 month follow up visit. A colonoscopy was performed 5 months after presentation and revealed normal descending colonic mucosa (Figure 2).
Discussion: Ischemic colitis is typically associated with risk factors for mesenteric ischemia. However, emerging evidence suggests that GLP-1 receptor agonists may lead to ischemic colitis. In this case, symptoms began shortly after a 5 day course of cephalexin, which has been rarely associated with antibiotic-associated colitis. However, the timing of tirzepatide initiation and resolution of symptoms following its discontinuation raises suspicion for tirzepatide-associated ischemic colitis. While causality cannot be definitively established, this case supports the need for clinical awareness of rare complications associated with Tirzepatide.
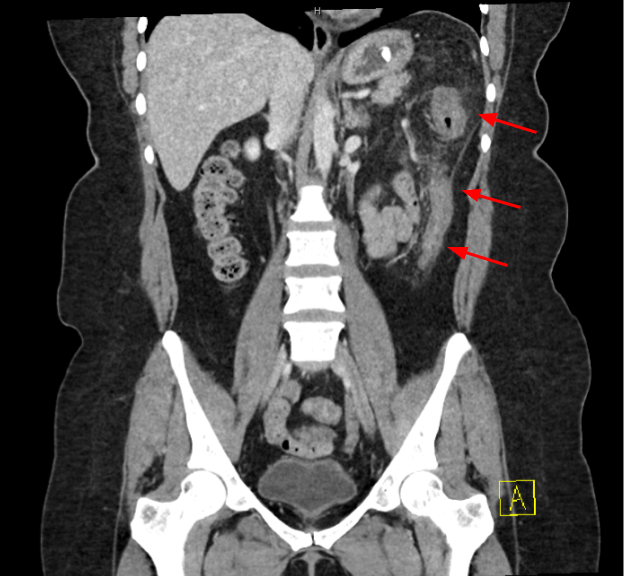
Figure: Figure 1: CT abdomen pelvis showing thickening of the left colon extending from the splenic flexure to the mid-descending colon with surrounding pericolonic fat haziness and stranding (arrows).
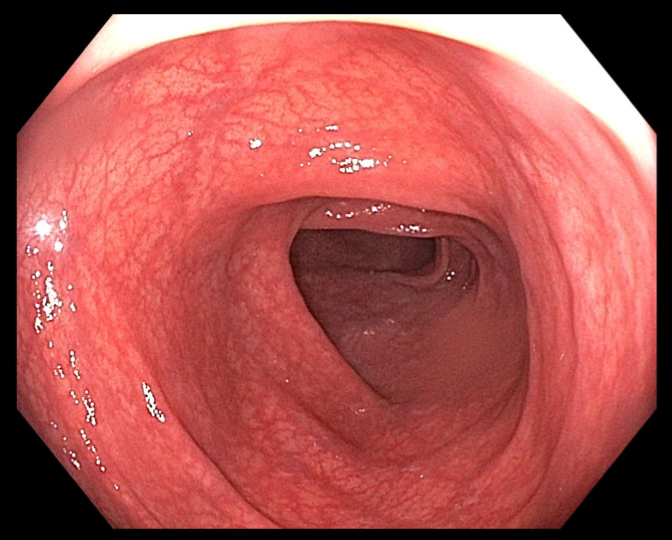
Figure: Figure 2: Image from follow-up colonoscopy showing the descending colon with normal colonic mucosa.
Disclosures:
Alexa Plato indicated no relevant financial relationships.
George Hanna indicated no relevant financial relationships.
Raquel Rozner indicated no relevant financial relationships.
Alexa Plato, MD1, George M. Hanna, MD1, Raquel Rozner, MD2. P4856 - Ischemic Colitis Following Tirzepatide Therapy: An Emerging Clinical Observation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Yale New Haven Health, Bridgeport Hospital, Bridgeport, CT; 2Yale School of Medicine, Gastroenterology Associates, PC, Northeast Medical Group/Yale New Haven Health, Stratford, CT
Introduction: Tirzepatide is a novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist approved for the treatment of type 2 diabetes mellitus and obesity. While its efficacy in glycemic control and weight loss is well established, its safety profile continues to evolve. Gastrointestinal side effects such as nausea, vomiting and diarrhea are common; however, serious adverse events involving the gastrointestinal tract, including ischemic colitis, have not been widely reported. Here we present a case of ischemic colitis temporally associated with the initiation of tirzepatide.
Case Description/
Methods: A 45-year-old female with a history of anxiety, depression and class II obesity presented to the emergency department with two days of nausea and painful hematochezia. She reported passing multiple blood clots but no stool. Her symptoms began 7 days after initiating a 5 day course of cephalexin for impetigo. She denied fevers, chills or prior endoscopic evaluation. There were no recent changes to her medications which included fluoxetine, lithium and lorazepam. Tirzepatide was initiated 8 months prior to presentation, with her last dose administered 5 days prior to presentation. The patient denied NSAID use, alcohol, tobacco or drug use. Laboratory results were notable for a C-reactive protein of 83.5 mg/L and white blood cell count of 11.6 x10⁹/L. CT scan demonstrated nonspecific colitis involving the descending colon (Figure 1). Based on the clinical presentation and imaging, ischemic colitis was suspected. Tirzepatide was held and she was treated with 7 days of antibiotics. The patient reported complete resolution of symptoms at her 1 month follow up visit. A colonoscopy was performed 5 months after presentation and revealed normal descending colonic mucosa (Figure 2).
Discussion: Ischemic colitis is typically associated with risk factors for mesenteric ischemia. However, emerging evidence suggests that GLP-1 receptor agonists may lead to ischemic colitis. In this case, symptoms began shortly after a 5 day course of cephalexin, which has been rarely associated with antibiotic-associated colitis. However, the timing of tirzepatide initiation and resolution of symptoms following its discontinuation raises suspicion for tirzepatide-associated ischemic colitis. While causality cannot be definitively established, this case supports the need for clinical awareness of rare complications associated with Tirzepatide.

Figure: Figure 1: CT abdomen pelvis showing thickening of the left colon extending from the splenic flexure to the mid-descending colon with surrounding pericolonic fat haziness and stranding (arrows).

Figure: Figure 2: Image from follow-up colonoscopy showing the descending colon with normal colonic mucosa.
Disclosures:
Alexa Plato indicated no relevant financial relationships.
George Hanna indicated no relevant financial relationships.
Raquel Rozner indicated no relevant financial relationships.
Alexa Plato, MD1, George M. Hanna, MD1, Raquel Rozner, MD2. P4856 - Ischemic Colitis Following Tirzepatide Therapy: An Emerging Clinical Observation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
