Tuesday Poster Session
Category: Diet, Nutrition, and Obesity
P4841 - Oral Peptide BPC-157 - An Emerging Adjunct to Gastrointestinal Therapies? A Systematic Review
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Nikhil Vasireddi, MD, MHA (he/him/his)
University of Iowa Hospitals & Clinics
Iowa City, IA
Presenting Author(s)
Nikhil Vasireddi, MD, MHA1, Neal Vasireddi, BS MEng2, Srinivas S. Vasireddi, MD3
1University of Iowa Hospitals & Clinics, Iowa City, IA; 2Robert Wood Johnson Medical School, Rutgers University, Holmdel, NJ; 3advanced digestive center Inc, Metuchen, NJ
Introduction: Body protection compound-157 (BPC-157) is a naturally occurring peptide endogenous to human gastric juice that promotes mucosal integrity and homeostasis. Preclinical studies show its cytoprotective and pro-healing effects throughout the gastrointestinal (GI) tract, including healing GI ulcers, anastomotic sites, various GI fistulas, as well as in models of inflammatory bowel disease. Despite lacking FDA approval, it is frequently sold online direct-to-consumer as a dietary supplement, and even offered by clinicians. Our objective is (1) to provide a comprehensive synthesis of the BPC-157 literature from a GI perspective and (2) to elucidate the mechanism of action, gastrointestinal outcomes, metabolism, and safety profile.
Methods: A systematic review of the English-language literature from PubMed, Cochrane, and Embase was conducted according to the PRISMA guidelines. Studies reporting BPC-157’s mechanism of action, gastrointestinal outcomes, metabolism, and safety were included.
Results: Thirty-six studies from 1993 to 2025 were included (Figure 1). BPC-157 enhances growth hormone receptor expression and modulates several pathways involved in cell growth and angiogenesis while reducing inflammatory cytokines (Figure 2). In preclinical models, BPC-157 improved functional and structural outcomes in inflammatory bowel disease, GI ulcer, NSAID induced injury, various GI fistula, and repaired anastomotic site models. BPC-157 is metabolized in the liver, with a half-life < 30 minutes, and is cleared by the kidneys. Preclinical safety studies showed no adverse effects across several organ systems. No clinical safety data is available to date.
Discussion: BPC-157 shows promise from pre-clinical studies for a range of GI pathologies—particularly in mucosal protection, wound healing, inflammatory bowel disease, and mitigation of NSAID-induced injury. Adverse effects are possible due to unregulated manufacturing, contamination, or unknown clinical safety, as it is not yet FDA approved, but available as a dietary supplement online. In summary, its use remains investigational, and it should not yet be considered a standard or evidence-based therapy for gastrointestinal diseases in clinical practice.
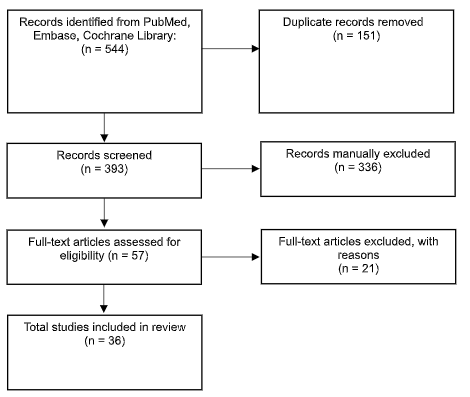
Figure: Figure 1. PRISMA Diagram.
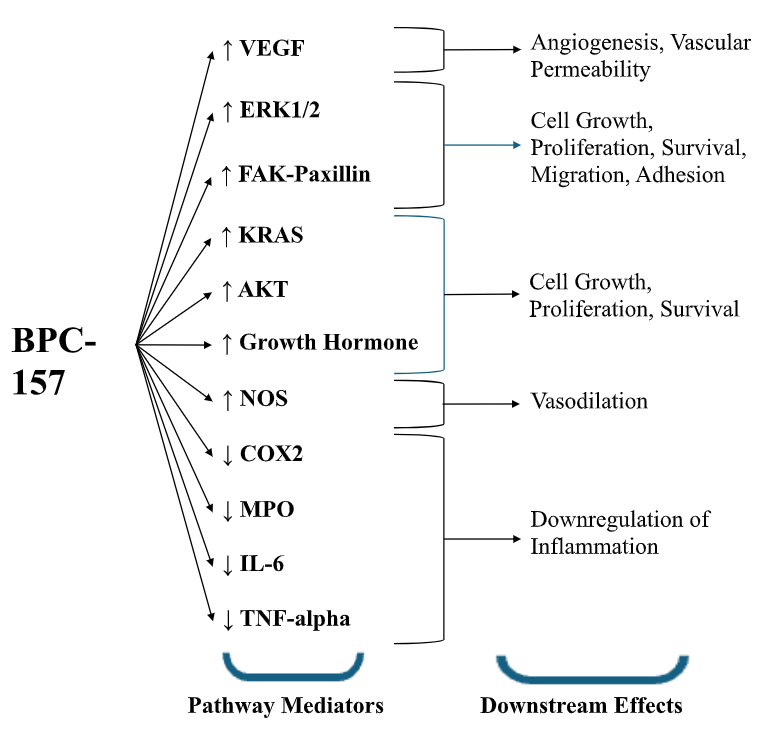
Figure: Figure 2. High level overview of pathways modulated by BPC-157. It is important to note that these pathways are nonlinear and often interconnected. VEGF, vascular endothelial growth factor; ERK1/2, extracellular signal-regulated kinase 1/2; FAK, focal adhesion kinase; KRAS, Kirsten rat sarcoma virus; AKT, alpha serine/threonine-protein kinase; NOS, nitric oxide synthase; COX2, cyclooxygenase-2; MPO, myeloperoxidase; IL-6, interleukin-6; TNF-alpha, tumor necrosis factor-alpha.
Disclosures:
Nikhil Vasireddi indicated no relevant financial relationships.
Neal Vasireddi indicated no relevant financial relationships.
Srinivas Vasireddi indicated no relevant financial relationships.
Nikhil Vasireddi, MD, MHA1, Neal Vasireddi, BS MEng2, Srinivas S. Vasireddi, MD3. P4841 - Oral Peptide BPC-157 - An Emerging Adjunct to Gastrointestinal Therapies? A Systematic Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Iowa Hospitals & Clinics, Iowa City, IA; 2Robert Wood Johnson Medical School, Rutgers University, Holmdel, NJ; 3advanced digestive center Inc, Metuchen, NJ
Introduction: Body protection compound-157 (BPC-157) is a naturally occurring peptide endogenous to human gastric juice that promotes mucosal integrity and homeostasis. Preclinical studies show its cytoprotective and pro-healing effects throughout the gastrointestinal (GI) tract, including healing GI ulcers, anastomotic sites, various GI fistulas, as well as in models of inflammatory bowel disease. Despite lacking FDA approval, it is frequently sold online direct-to-consumer as a dietary supplement, and even offered by clinicians. Our objective is (1) to provide a comprehensive synthesis of the BPC-157 literature from a GI perspective and (2) to elucidate the mechanism of action, gastrointestinal outcomes, metabolism, and safety profile.
Methods: A systematic review of the English-language literature from PubMed, Cochrane, and Embase was conducted according to the PRISMA guidelines. Studies reporting BPC-157’s mechanism of action, gastrointestinal outcomes, metabolism, and safety were included.
Results: Thirty-six studies from 1993 to 2025 were included (Figure 1). BPC-157 enhances growth hormone receptor expression and modulates several pathways involved in cell growth and angiogenesis while reducing inflammatory cytokines (Figure 2). In preclinical models, BPC-157 improved functional and structural outcomes in inflammatory bowel disease, GI ulcer, NSAID induced injury, various GI fistula, and repaired anastomotic site models. BPC-157 is metabolized in the liver, with a half-life < 30 minutes, and is cleared by the kidneys. Preclinical safety studies showed no adverse effects across several organ systems. No clinical safety data is available to date.
Discussion: BPC-157 shows promise from pre-clinical studies for a range of GI pathologies—particularly in mucosal protection, wound healing, inflammatory bowel disease, and mitigation of NSAID-induced injury. Adverse effects are possible due to unregulated manufacturing, contamination, or unknown clinical safety, as it is not yet FDA approved, but available as a dietary supplement online. In summary, its use remains investigational, and it should not yet be considered a standard or evidence-based therapy for gastrointestinal diseases in clinical practice.

Figure: Figure 1. PRISMA Diagram.

Figure: Figure 2. High level overview of pathways modulated by BPC-157. It is important to note that these pathways are nonlinear and often interconnected. VEGF, vascular endothelial growth factor; ERK1/2, extracellular signal-regulated kinase 1/2; FAK, focal adhesion kinase; KRAS, Kirsten rat sarcoma virus; AKT, alpha serine/threonine-protein kinase; NOS, nitric oxide synthase; COX2, cyclooxygenase-2; MPO, myeloperoxidase; IL-6, interleukin-6; TNF-alpha, tumor necrosis factor-alpha.
Disclosures:
Nikhil Vasireddi indicated no relevant financial relationships.
Neal Vasireddi indicated no relevant financial relationships.
Srinivas Vasireddi indicated no relevant financial relationships.
Nikhil Vasireddi, MD, MHA1, Neal Vasireddi, BS MEng2, Srinivas S. Vasireddi, MD3. P4841 - Oral Peptide BPC-157 - An Emerging Adjunct to Gastrointestinal Therapies? A Systematic Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

