Tuesday Poster Session
Category: Diet, Nutrition, and Obesity
P4832 - Gastrointestial Outcomes in Patients With Concomitant Use of Immuno Checkpoint Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists: A Population-Based Cohort Study in the United States
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- LN
Luis M. Nieto, MD (he/him/his)
Emory University School of Medicine
Atlanta, GA
Presenting Author(s)
Luis M.. Nieto, MD1, Sharon I.. Narvaez, MD2, Kimia Badakhshan, MS3, Donghyun Ko, MD4, Do Han Kim, MD, MSc5, Olanrewaju Adeniran, MD6, Saurabh Chawla, MD, FACG1, Steven Keilin, MD7, Kenneth J.. Vega, MD, MHA8
1Emory University School of Medicine, Atlanta, GA; 2Piedmont Athens Regional, Atlanta, GA; 3Philadelphia College of Osteopathic Medicine, Longwood, FL; 4Bridgeport Hospital, Bridgeport, CT; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6West Virginia University Morgantown, Morgantown, WV; 7Emory University Hospital, Atlanta, GA; 8Prisma Health Midlands, Columbia, SC
Introduction: The use of immune checkpoint inhibitors (ICIs) to treat advanced cancers is growing. They may result in a number of gastrointestinal (GI) adverse effects. There is no data on the GI outcomes in patients who are taking Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), which is also a drug with GI side effects, along with ICIs. Therefore, we aim to evaluate the GI outcomes and all-cause mortality in the patient population taking those drugs.
Methods: This is a retrospective cohort study using a large population-based data from the TriNetX platform. We included patients with who received GLP-1 RAs drugs (semaglutide, liraglutide, dulaglutide and tirzepatide) and ICIs drugs (ipilimumab, nivolumab, pembrolizumab, atezolizumab, atezolizumab, avelumab, durvalumab, relatlimab and cemiplimab) between January 1, 2010, and October 31, 2024. This cohort of patients were matched with patients who did not receive GLP-1 RAs but received ICIs drugs according to age, demographics, comorbidities, and medication by using 1:1 propensity matching. Primary outcomes included the oods of developing diarrhea, colitis, nausea and vomiting, early satiety, bloating, GERD, esophagitis, gastritis and duodenitis, hepatitis and pancreatitis. The secondary outcome was all-cause mortality. Log regression models were used to estimate odds ratios (ORs).
Results: A total of 124,750 patients using ICIs were identified, 4,155 of these individuals were on GLP -1 RAs; 4,148 out of those 4,155 (mean [SD] age, 64.2 [11.4] years; 1,905 [45.9%] female) were matched with 4,148 individuals (mean [SD] age, 64.3[12.9] years; 1,884 [45.4%] female) who did not take GLP-1 RAs. GLP-1 RAs was associated with lower odds of all-cause mortality (OR, 0.37; 95% CI, 0.34-0.41), diarrhea (OR, 0.83; 95% CI, 0.77-0.96), colitis (OR, 0.75; 95% CI, 0.64-0.88), nausea and vomiting (OR, 0.83; 95% CI, 0.72-0.95), early satiety (OR, 0.52; 95% CI, 0.31-0.87), bloating (OR, 0.77; 95% CI, 0.62-0.95), and esophagitis (OR, 0.73; 95% CI, 0.54-0.97), when compared with non-GLP-1RAs use. No difference was found on GERD, gastritis and duodenitis, hepatitis and pancreatitis when compared GLP-1 vs non-GLP-1.
Discussion: Based on our findings, concomitant use of GLP-1 RAs and ICIs is associated with lower odds for diarrhea, colitis, nausea and vomiting, early satiety, bloating and esophagitis. Also, using both medications do not increase the risk for GERD, hepatitis or pancreatitis. Further prospective studies are needed to better understand these findings.
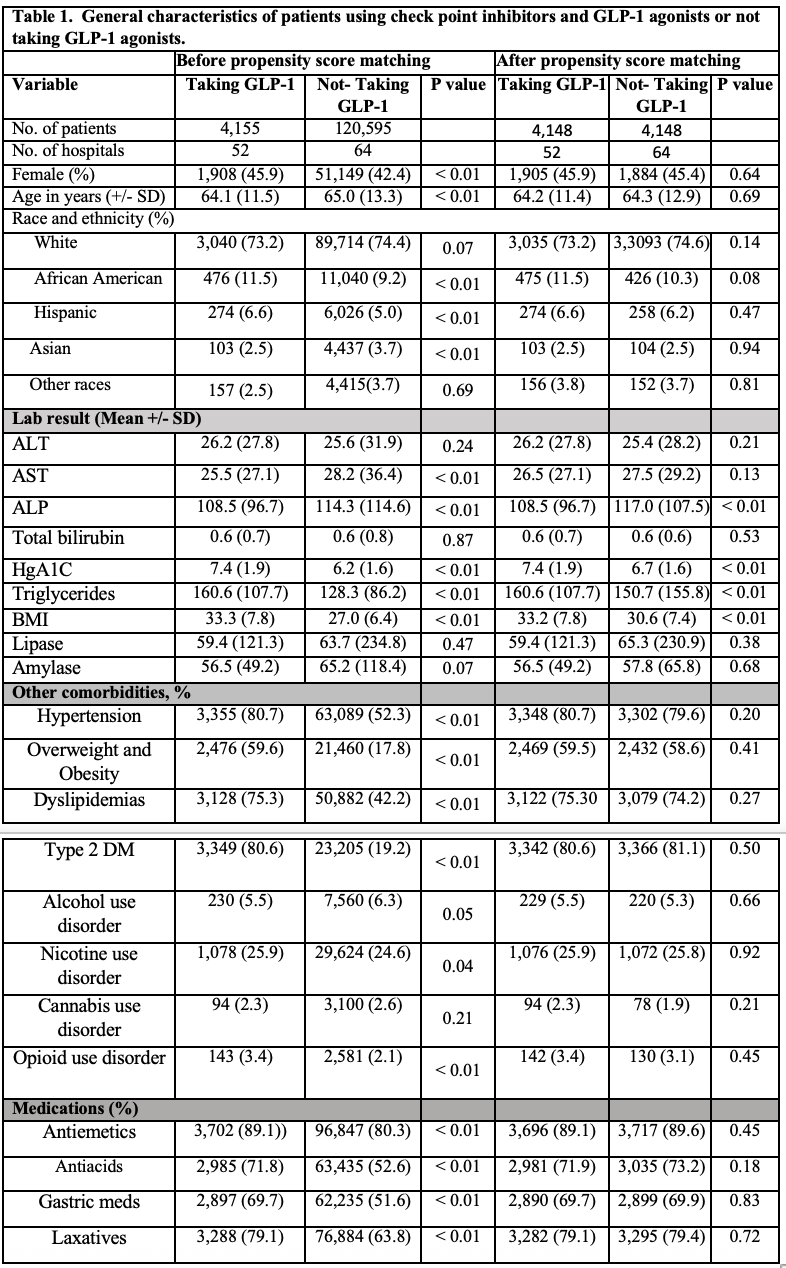
Figure: General characteristics of patients using check point inhibitors and GLP-1 agonists or not taking GLP-1 agonists.
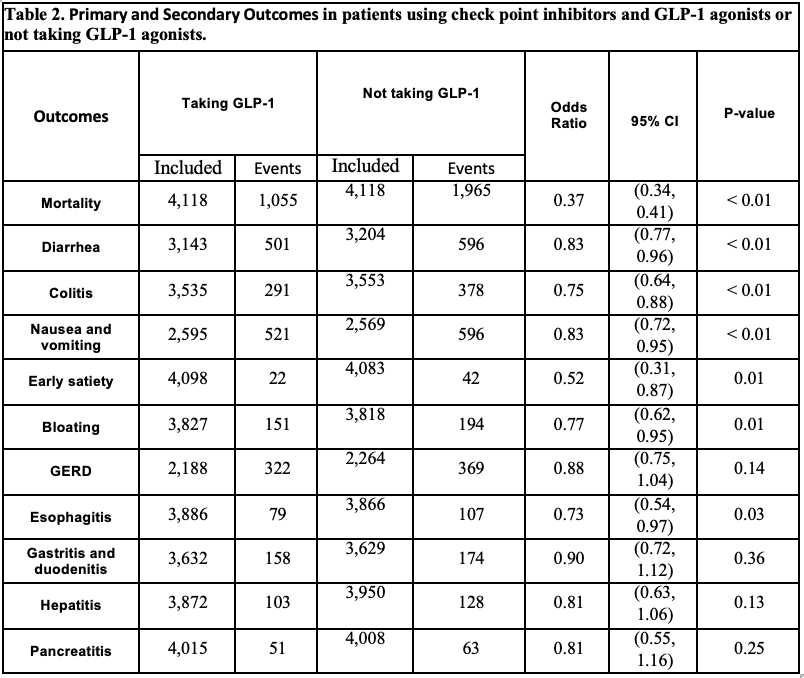
Figure: Primary and Secondary Outcomes in patients using check point inhibitors and GLP-1 agonists or not taking GLP-1 agonists.
Disclosures:
Luis Nieto indicated no relevant financial relationships.
Sharon Narvaez indicated no relevant financial relationships.
Kimia Badakhshan indicated no relevant financial relationships.
Donghyun Ko indicated no relevant financial relationships.
Do Han Kim indicated no relevant financial relationships.
Olanrewaju Adeniran indicated no relevant financial relationships.
Saurabh Chawla indicated no relevant financial relationships.
Steven Keilin indicated no relevant financial relationships.
Kenneth Vega: Phathom Pharmceuticals – Speakers Bureau.
Luis M.. Nieto, MD1, Sharon I.. Narvaez, MD2, Kimia Badakhshan, MS3, Donghyun Ko, MD4, Do Han Kim, MD, MSc5, Olanrewaju Adeniran, MD6, Saurabh Chawla, MD, FACG1, Steven Keilin, MD7, Kenneth J.. Vega, MD, MHA8. P4832 - Gastrointestial Outcomes in Patients With Concomitant Use of Immuno Checkpoint Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists: A Population-Based Cohort Study in the United States, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Emory University School of Medicine, Atlanta, GA; 2Piedmont Athens Regional, Atlanta, GA; 3Philadelphia College of Osteopathic Medicine, Longwood, FL; 4Bridgeport Hospital, Bridgeport, CT; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6West Virginia University Morgantown, Morgantown, WV; 7Emory University Hospital, Atlanta, GA; 8Prisma Health Midlands, Columbia, SC
Introduction: The use of immune checkpoint inhibitors (ICIs) to treat advanced cancers is growing. They may result in a number of gastrointestinal (GI) adverse effects. There is no data on the GI outcomes in patients who are taking Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), which is also a drug with GI side effects, along with ICIs. Therefore, we aim to evaluate the GI outcomes and all-cause mortality in the patient population taking those drugs.
Methods: This is a retrospective cohort study using a large population-based data from the TriNetX platform. We included patients with who received GLP-1 RAs drugs (semaglutide, liraglutide, dulaglutide and tirzepatide) and ICIs drugs (ipilimumab, nivolumab, pembrolizumab, atezolizumab, atezolizumab, avelumab, durvalumab, relatlimab and cemiplimab) between January 1, 2010, and October 31, 2024. This cohort of patients were matched with patients who did not receive GLP-1 RAs but received ICIs drugs according to age, demographics, comorbidities, and medication by using 1:1 propensity matching. Primary outcomes included the oods of developing diarrhea, colitis, nausea and vomiting, early satiety, bloating, GERD, esophagitis, gastritis and duodenitis, hepatitis and pancreatitis. The secondary outcome was all-cause mortality. Log regression models were used to estimate odds ratios (ORs).
Results: A total of 124,750 patients using ICIs were identified, 4,155 of these individuals were on GLP -1 RAs; 4,148 out of those 4,155 (mean [SD] age, 64.2 [11.4] years; 1,905 [45.9%] female) were matched with 4,148 individuals (mean [SD] age, 64.3[12.9] years; 1,884 [45.4%] female) who did not take GLP-1 RAs. GLP-1 RAs was associated with lower odds of all-cause mortality (OR, 0.37; 95% CI, 0.34-0.41), diarrhea (OR, 0.83; 95% CI, 0.77-0.96), colitis (OR, 0.75; 95% CI, 0.64-0.88), nausea and vomiting (OR, 0.83; 95% CI, 0.72-0.95), early satiety (OR, 0.52; 95% CI, 0.31-0.87), bloating (OR, 0.77; 95% CI, 0.62-0.95), and esophagitis (OR, 0.73; 95% CI, 0.54-0.97), when compared with non-GLP-1RAs use. No difference was found on GERD, gastritis and duodenitis, hepatitis and pancreatitis when compared GLP-1 vs non-GLP-1.
Discussion: Based on our findings, concomitant use of GLP-1 RAs and ICIs is associated with lower odds for diarrhea, colitis, nausea and vomiting, early satiety, bloating and esophagitis. Also, using both medications do not increase the risk for GERD, hepatitis or pancreatitis. Further prospective studies are needed to better understand these findings.

Figure: General characteristics of patients using check point inhibitors and GLP-1 agonists or not taking GLP-1 agonists.

Figure: Primary and Secondary Outcomes in patients using check point inhibitors and GLP-1 agonists or not taking GLP-1 agonists.
Disclosures:
Luis Nieto indicated no relevant financial relationships.
Sharon Narvaez indicated no relevant financial relationships.
Kimia Badakhshan indicated no relevant financial relationships.
Donghyun Ko indicated no relevant financial relationships.
Do Han Kim indicated no relevant financial relationships.
Olanrewaju Adeniran indicated no relevant financial relationships.
Saurabh Chawla indicated no relevant financial relationships.
Steven Keilin indicated no relevant financial relationships.
Kenneth Vega: Phathom Pharmceuticals – Speakers Bureau.
Luis M.. Nieto, MD1, Sharon I.. Narvaez, MD2, Kimia Badakhshan, MS3, Donghyun Ko, MD4, Do Han Kim, MD, MSc5, Olanrewaju Adeniran, MD6, Saurabh Chawla, MD, FACG1, Steven Keilin, MD7, Kenneth J.. Vega, MD, MHA8. P4832 - Gastrointestial Outcomes in Patients With Concomitant Use of Immuno Checkpoint Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists: A Population-Based Cohort Study in the United States, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
