Tuesday Poster Session
Category: Diet, Nutrition, and Obesity
P4830 - Gastrointestinal Adverse Events of Glucagon-Like Peptide-1 Receptor Agonists Used for Weight Loss: A Real-World Cohort Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Saqr Alsakarneh, MD, MS (he/him/his)
Mayo Clinic
Rochester, MN
Presenting Author(s)
Saqr Alsakarneh, MD, MS1, Omar Al Ta’ani, MD2, Razan Aburumman, MD3, Farah Al-Bitar, MD4, Francis A.. Farraye, MD, MSc, MACG5, Jana G. Hashash, MD, MSc, FACG5
1Mayo Clinic, Kansas City, MO; 2Department of Internal Medicine, Allegheny Health Network, Pittsburgh, Pennsylvania, USA, Pittsburgh, PA; 3Henry Ford Health, Detroit, MI; 4Western Michigan University, Kalamazoo, MI; 5Mayo Clinic, Jacksonville, FL
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and naltrexone-bupropion are commonly prescribed for weight management. While GLP-1RAs offer superior weight loss, emerging evidence links them to serious gastrointestinal (GI) complications. Existing safety data are limited by small trials and short follow-up. We aimed to compare the incidence of GI adverse events in patients with obesity treated with GLP-1RAs versus naltrexone-bupropion.
Methods: We conducted a retrospective cohort study using the TriNetX database to identify adults (≥18 years) with obesity. We then identified patients who were treated with GLP-1RA for weight loss. Patients in the GLP-1RA cohort were matched to patients used naltrexone-bupropion for weight loss using 1:1 propensity score matching for demographics, comorbidities, and geographical location (Table 1). Primary outcome was the incidence of GI adverse events including biliary disease, pancreatitis, bowel obstruction, and gastroparesis. Kaplan-Meier analysis with Hazard ratios (HRs) and 95% confidence intervals were calculated using Cox proportional hazards models.
Results: Among 8,903 patients treated with GLP-1RAs, the mean age was 46.4 ± 13.2 years, and 74.3% were females. Compared to bupropion-naltrexone, GLP-1RA use was associated with an increased risk of gastroparesis (aHR: 3.13, 95% CI: 1.82–5.38; p < 0.001) and gallbladder disease (aHR: 1.18, 95% CI: 1.02–1.37; p = 0.027) (Table 2). No significant association was observed for acute pancreatitis (aHR: 1.42, 95% CI: 0.88–2.29; p = 0.153) or intestinal obstruction (aHR: 0.98, 95% CI: 0.71–1.35; p = 0.891). Subgroup analyses revealed that the highest risk of gastroparesis was in patients aged >50 years (aHR: 2.21, 95% CI: 1.21–4.04; p = 0.008). The association with gallbladder disease was strongest in females (aHR: 1.36, 95% CI: 1.15–1.60; p = < 0.001) and adults under 50 (aHR: 1.27, 95% CI: 1.05–1.53; p = 0.014) [Table 2].
Discussion: Our results provide real-world evidence that GLP-1RAs were associated with higher risks of gastroparesis and gallbladder disease compared to naltrexone-bupropion in patients with obesity. Although these adverse events are relatively uncommon, their potential clinical impact warrants attention. This is particularly important for individuals using GLP-1RAs exclusively for weight loss, where the therapeutic risk-benefit profile may differ from those with type 2 diabetes. Clinicians should communicate these risks to support individualized decisions in obesity pharmacotherapy.
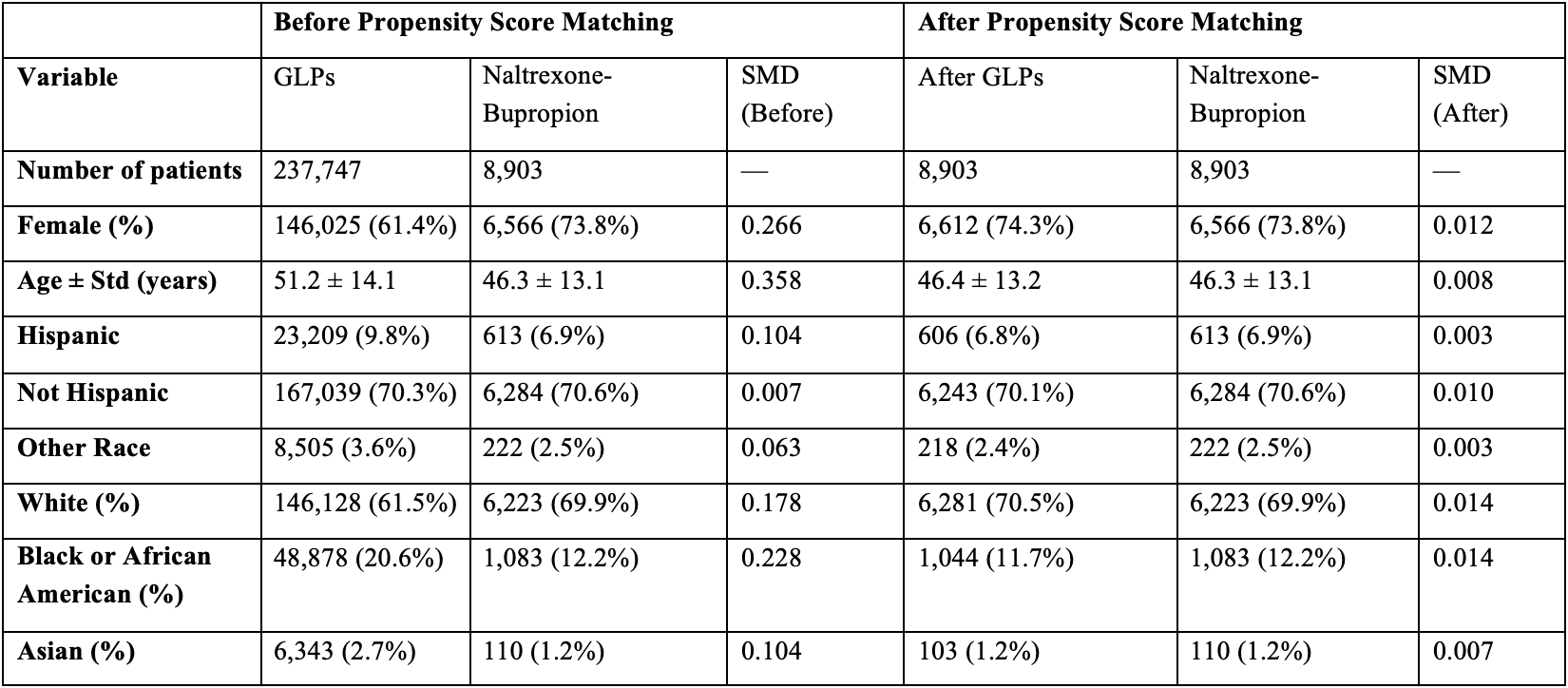
Figure: Table 1. Baseline Demographic Characteristics of Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and naltrexone-bupropion Cohorts Before and After Propensity Score Matching
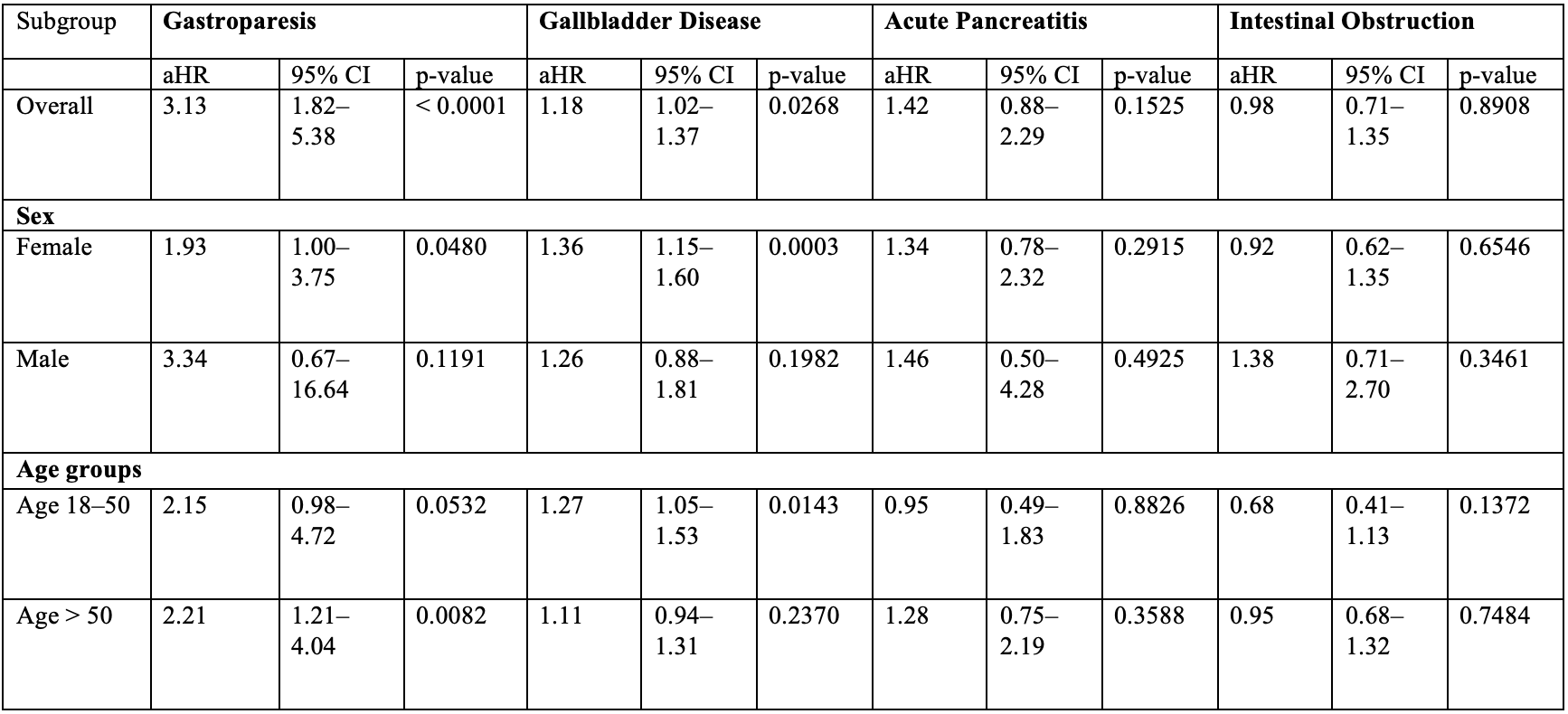
Figure: Table 2. Gastrointestinal Adverse Events Associated with GLP-1 Receptor Agonists.
Disclosures:
Saqr Alsakarneh indicated no relevant financial relationships.
Omar Al Ta’ani indicated no relevant financial relationships.
Razan Aburumman indicated no relevant financial relationships.
Farah Al-Bitar indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Jana Hashash: BMS – Ad Board.
Saqr Alsakarneh, MD, MS1, Omar Al Ta’ani, MD2, Razan Aburumman, MD3, Farah Al-Bitar, MD4, Francis A.. Farraye, MD, MSc, MACG5, Jana G. Hashash, MD, MSc, FACG5. P4830 - Gastrointestinal Adverse Events of Glucagon-Like Peptide-1 Receptor Agonists Used for Weight Loss: A Real-World Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Kansas City, MO; 2Department of Internal Medicine, Allegheny Health Network, Pittsburgh, Pennsylvania, USA, Pittsburgh, PA; 3Henry Ford Health, Detroit, MI; 4Western Michigan University, Kalamazoo, MI; 5Mayo Clinic, Jacksonville, FL
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and naltrexone-bupropion are commonly prescribed for weight management. While GLP-1RAs offer superior weight loss, emerging evidence links them to serious gastrointestinal (GI) complications. Existing safety data are limited by small trials and short follow-up. We aimed to compare the incidence of GI adverse events in patients with obesity treated with GLP-1RAs versus naltrexone-bupropion.
Methods: We conducted a retrospective cohort study using the TriNetX database to identify adults (≥18 years) with obesity. We then identified patients who were treated with GLP-1RA for weight loss. Patients in the GLP-1RA cohort were matched to patients used naltrexone-bupropion for weight loss using 1:1 propensity score matching for demographics, comorbidities, and geographical location (Table 1). Primary outcome was the incidence of GI adverse events including biliary disease, pancreatitis, bowel obstruction, and gastroparesis. Kaplan-Meier analysis with Hazard ratios (HRs) and 95% confidence intervals were calculated using Cox proportional hazards models.
Results: Among 8,903 patients treated with GLP-1RAs, the mean age was 46.4 ± 13.2 years, and 74.3% were females. Compared to bupropion-naltrexone, GLP-1RA use was associated with an increased risk of gastroparesis (aHR: 3.13, 95% CI: 1.82–5.38; p < 0.001) and gallbladder disease (aHR: 1.18, 95% CI: 1.02–1.37; p = 0.027) (Table 2). No significant association was observed for acute pancreatitis (aHR: 1.42, 95% CI: 0.88–2.29; p = 0.153) or intestinal obstruction (aHR: 0.98, 95% CI: 0.71–1.35; p = 0.891). Subgroup analyses revealed that the highest risk of gastroparesis was in patients aged >50 years (aHR: 2.21, 95% CI: 1.21–4.04; p = 0.008). The association with gallbladder disease was strongest in females (aHR: 1.36, 95% CI: 1.15–1.60; p = < 0.001) and adults under 50 (aHR: 1.27, 95% CI: 1.05–1.53; p = 0.014) [Table 2].
Discussion: Our results provide real-world evidence that GLP-1RAs were associated with higher risks of gastroparesis and gallbladder disease compared to naltrexone-bupropion in patients with obesity. Although these adverse events are relatively uncommon, their potential clinical impact warrants attention. This is particularly important for individuals using GLP-1RAs exclusively for weight loss, where the therapeutic risk-benefit profile may differ from those with type 2 diabetes. Clinicians should communicate these risks to support individualized decisions in obesity pharmacotherapy.

Figure: Table 1. Baseline Demographic Characteristics of Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and naltrexone-bupropion Cohorts Before and After Propensity Score Matching

Figure: Table 2. Gastrointestinal Adverse Events Associated with GLP-1 Receptor Agonists.
Disclosures:
Saqr Alsakarneh indicated no relevant financial relationships.
Omar Al Ta’ani indicated no relevant financial relationships.
Razan Aburumman indicated no relevant financial relationships.
Farah Al-Bitar indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Jana Hashash: BMS – Ad Board.
Saqr Alsakarneh, MD, MS1, Omar Al Ta’ani, MD2, Razan Aburumman, MD3, Farah Al-Bitar, MD4, Francis A.. Farraye, MD, MSc, MACG5, Jana G. Hashash, MD, MSc, FACG5. P4830 - Gastrointestinal Adverse Events of Glucagon-Like Peptide-1 Receptor Agonists Used for Weight Loss: A Real-World Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
