Tuesday Poster Session
Category: Diet, Nutrition, and Obesity
P4821 - Oral Super-Absorbent Hydrogel Capsules Double Clinically Meaningful Weight Loss Without Added Serious or GI Adverse Events: A Systematic Review and Meta Analysis of Randomized Controlled Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Ashesh Das, MBBS
KPC Medical College and Hospital , Kolkata, India
Kolkata, West Bengal, India
Presenting Author(s)
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, M Uzair Tahir, MBBS3, Noorul Hidhaya, MBBS4, Urvashi Bharia, 5, Mustafa Al Jnainati, MD6, Fazia Khattak, 7, Emil Vergis Philip, MBBS8, Mohamed Nasser Elshabrawi, MBBS9
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 3King Edward Medical University, Lahore, Punjab, Pakistan; 4Stanley Medical College, Chennai, Tamil Nadu, India; 5Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai, Navi Mumbai, Maharashtra, India; 6University of Bologna, Bologna, Emilia-Romagna, Italy; 7Khyber Medical College, Peshawar, North-West Frontier, Pakistan; 8Madras Medical College, Kottayam, Kerala, India; 9Port Said university, Port Said, Al Isma'iliyah, Egypt
Introduction: The obesity epidemic continues to outpace the capacity of pharmacologic and endoscopic therapies, many of which carry systemic effects, high cost, or procedural risk. Oral super-absorbent hydrogel (OSH) capsules—an ingestible, non-systemic biomaterial that swells in the stomach to promote satiety—were recently cleared by the FDA yet remain under-recognized in gastroenterology practice. By taking three placebo-controlled Randomized Controlled Trials (RCTs) with a total of 990 participants, we aimed to provide the first high-certainty estimate of OSH-mediated weight loss and to clarify its safety profile.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified RCTs that compares the efficacy and safety of OSH versus placebo for weight loss through May 2025. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Across three RCTs (n=990) with overweight or obesity, oral super-absorbent hydrogel capsules (n = 501) achieved superior efficacy and comparable safety versus placebo: ≥5 % weight loss occurred in 54 % vs 36 % of participants (RR 1.54, 95 % CI 1.16–2.03; I² = 70 %), and ≥10 % weight loss in 24 % vs 11 % (RR 2.24, 95 % CI 1.66–3.02; I² = 30 %); no device-related serious adverse events were reported and overall SAEs were rare (0/501 vs 4/489; RR 0.19, 95 % CI 0.02–1.65), while gastrointestinal side-effects (31 % vs 27 %; RR 1.11, 95 % CI 0.82–1.50; I² = 54 %) and discontinuations for adverse events (1.6 % vs 2.2 %; RR 0.71, 95 % CI 0.29–1.76; I² = 0 %) were similar.
Discussion: Our analysis shows that OSH nearly doubles the likelihood of achieving ≥10 % body-weight loss within 24 weeks while preserving a placebo-like rate of serious or treatment-limiting adverse events—an efficacy-to-safety balance that rivals early experience with GLP-1 analogues but without systemic exposure. Although moderate heterogeneity was observed for the ≥5 % threshold, effect direction was uniformly favorable, and no single trial drove the result, underscoring the robustness of the finding. These data position OSH as a practical, non-pharmacologic adjunct for patients who are refuse drug or bariatric interventions, and they suggest a role in mitigating obesity-linked GI comorbidities (e.g., NAFLD, GERD).
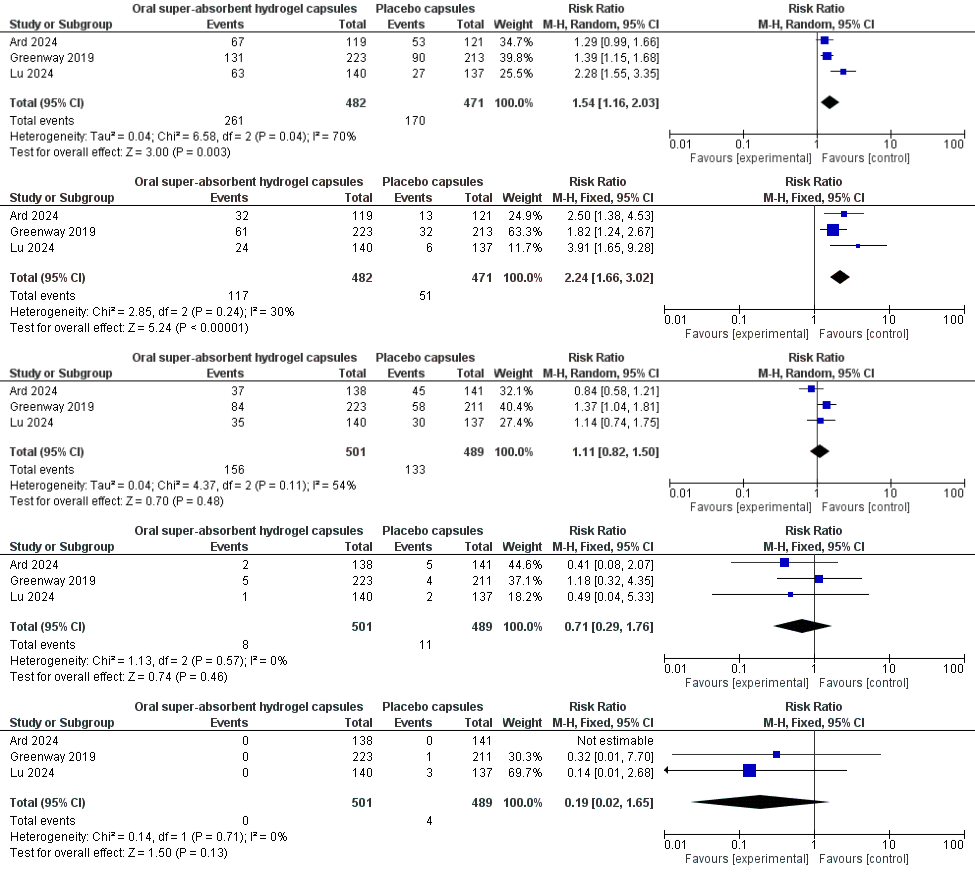
Figure: Forest Plot Showing ≥ 5% Body-Weight Loss from Baseline at 12–24 Weeks, ≥ 10% Body-Weight Loss at 24weeks, Any Treatment Emergent Gastrointestinal Side Effects, Discontinuation due to Adverse Events, Serious Adverse Events
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
M Uzair Tahir indicated no relevant financial relationships.
Noorul Hidhaya indicated no relevant financial relationships.
Urvashi Bharia indicated no relevant financial relationships.
Mustafa Al Jnainati indicated no relevant financial relationships.
Fazia Khattak indicated no relevant financial relationships.
Emil Vergis Philip indicated no relevant financial relationships.
Mohamed Nasser Elshabrawi indicated no relevant financial relationships.
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, M Uzair Tahir, MBBS3, Noorul Hidhaya, MBBS4, Urvashi Bharia, 5, Mustafa Al Jnainati, MD6, Fazia Khattak, 7, Emil Vergis Philip, MBBS8, Mohamed Nasser Elshabrawi, MBBS9. P4821 - Oral Super-Absorbent Hydrogel Capsules Double Clinically Meaningful Weight Loss Without Added Serious or GI Adverse Events: A Systematic Review and Meta Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 3King Edward Medical University, Lahore, Punjab, Pakistan; 4Stanley Medical College, Chennai, Tamil Nadu, India; 5Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai, Navi Mumbai, Maharashtra, India; 6University of Bologna, Bologna, Emilia-Romagna, Italy; 7Khyber Medical College, Peshawar, North-West Frontier, Pakistan; 8Madras Medical College, Kottayam, Kerala, India; 9Port Said university, Port Said, Al Isma'iliyah, Egypt
Introduction: The obesity epidemic continues to outpace the capacity of pharmacologic and endoscopic therapies, many of which carry systemic effects, high cost, or procedural risk. Oral super-absorbent hydrogel (OSH) capsules—an ingestible, non-systemic biomaterial that swells in the stomach to promote satiety—were recently cleared by the FDA yet remain under-recognized in gastroenterology practice. By taking three placebo-controlled Randomized Controlled Trials (RCTs) with a total of 990 participants, we aimed to provide the first high-certainty estimate of OSH-mediated weight loss and to clarify its safety profile.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified RCTs that compares the efficacy and safety of OSH versus placebo for weight loss through May 2025. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Across three RCTs (n=990) with overweight or obesity, oral super-absorbent hydrogel capsules (n = 501) achieved superior efficacy and comparable safety versus placebo: ≥5 % weight loss occurred in 54 % vs 36 % of participants (RR 1.54, 95 % CI 1.16–2.03; I² = 70 %), and ≥10 % weight loss in 24 % vs 11 % (RR 2.24, 95 % CI 1.66–3.02; I² = 30 %); no device-related serious adverse events were reported and overall SAEs were rare (0/501 vs 4/489; RR 0.19, 95 % CI 0.02–1.65), while gastrointestinal side-effects (31 % vs 27 %; RR 1.11, 95 % CI 0.82–1.50; I² = 54 %) and discontinuations for adverse events (1.6 % vs 2.2 %; RR 0.71, 95 % CI 0.29–1.76; I² = 0 %) were similar.
Discussion: Our analysis shows that OSH nearly doubles the likelihood of achieving ≥10 % body-weight loss within 24 weeks while preserving a placebo-like rate of serious or treatment-limiting adverse events—an efficacy-to-safety balance that rivals early experience with GLP-1 analogues but without systemic exposure. Although moderate heterogeneity was observed for the ≥5 % threshold, effect direction was uniformly favorable, and no single trial drove the result, underscoring the robustness of the finding. These data position OSH as a practical, non-pharmacologic adjunct for patients who are refuse drug or bariatric interventions, and they suggest a role in mitigating obesity-linked GI comorbidities (e.g., NAFLD, GERD).

Figure: Forest Plot Showing ≥ 5% Body-Weight Loss from Baseline at 12–24 Weeks, ≥ 10% Body-Weight Loss at 24weeks, Any Treatment Emergent Gastrointestinal Side Effects, Discontinuation due to Adverse Events, Serious Adverse Events
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
M Uzair Tahir indicated no relevant financial relationships.
Noorul Hidhaya indicated no relevant financial relationships.
Urvashi Bharia indicated no relevant financial relationships.
Mustafa Al Jnainati indicated no relevant financial relationships.
Fazia Khattak indicated no relevant financial relationships.
Emil Vergis Philip indicated no relevant financial relationships.
Mohamed Nasser Elshabrawi indicated no relevant financial relationships.
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, M Uzair Tahir, MBBS3, Noorul Hidhaya, MBBS4, Urvashi Bharia, 5, Mustafa Al Jnainati, MD6, Fazia Khattak, 7, Emil Vergis Philip, MBBS8, Mohamed Nasser Elshabrawi, MBBS9. P4821 - Oral Super-Absorbent Hydrogel Capsules Double Clinically Meaningful Weight Loss Without Added Serious or GI Adverse Events: A Systematic Review and Meta Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
