Tuesday Poster Session
Category: Liver
P6061 - Hepatocellular Adenoma Associated With Testosterone-Based Gender-Affirming Hormone Therapy in a Transgender Man
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Thanh Nguyen, MD
1. Department of Internal Medicine, Highland Hospital, Oakland, CA, USA
Oakland, CA
Presenting Author(s)
Thanh Nguyen, MD1, Tzu-Hao Lee, MD2
11. Department of Internal Medicine, Highland Hospital, Oakland, CA, USA, Oakland, CA; 22. Section of Gastroenterology and Hepatology, Department of Medicine, Baylor College of Medicine, Houston, TX, USA., Houston, TX
Introduction:
Introduction: Transgender and gender-diverse (TGD) individuals may receive gender-affirming hormone therapy (GAHT), testosterone for transgender men and estradiol for transgender women, to align their physical appearance with gender identity. While GAHT is generally safe, rare associations between long-term testosterone use and hepatobiliary neoplasms, particularly hepatocellular adenoma in transgender men, have been reported. We describe a case of hepatocellular adenoma in a transgender man likely linked to prolonged testosterone therapy.
Case Description/
Methods:
Case Presentation: A 19-year-old transgender man on transdermal testosterone and norethindrone for four years presented with chronic abdominal cramping. His medical history included dyslipidemia (treated with atorvastatin) and new persistent liver enzyme elevations over the preceding six months. He denied alcohol and tobacco use; family history was unremarkable. Physical examination revealed a BMI of 33 kg/m² with otherwise unremarkable exam. Laboratory studies showed elevated ALT (121 U/L), AST (44 U/L), and ALP (148 U/L); normal bilirubin (0.4 mg/dL), albumin (4.7 g/dL), platelets (363 × 10³/µL), PT/INR, and CRP; and a positive ANA (1:160) with negative anti–smooth muscle antibody and negative viral hepatitis serologies. An abdominal ultrasound performed 18 months prior had shown increased hepatic echogenicity without focal lesions. Current MRI identified three liver masses, the largest measuring 6 cm in segment 8. Percutaneous biopsy confirmed hepatocellular adenoma. Testosterone therapy was held, and plans were made for close follow-up with interval imaging.
Discussion:
Discussion: Hepatocellular adenomas in transgender men on testosterone-based GAHT are rare, with fewer than five cases reported to date, and progestins appear to play a negligible role in this context. Because uninterrupted access to GAHT is vital for many TGD individuals, a multidisciplinary approach is critical to enable prompt identification and management of hepatic lesions while minimizing interruptions to GAHT. Clinicians should remain vigilant for potential liver complications in TGD patients receiving testosterone, ensuring that hormone therapy can continue safely whenever feasible. Larger cohort studies and dedicated registries are needed to determine the true incidence and risk factors for hepatobiliary neoplasms in this population.
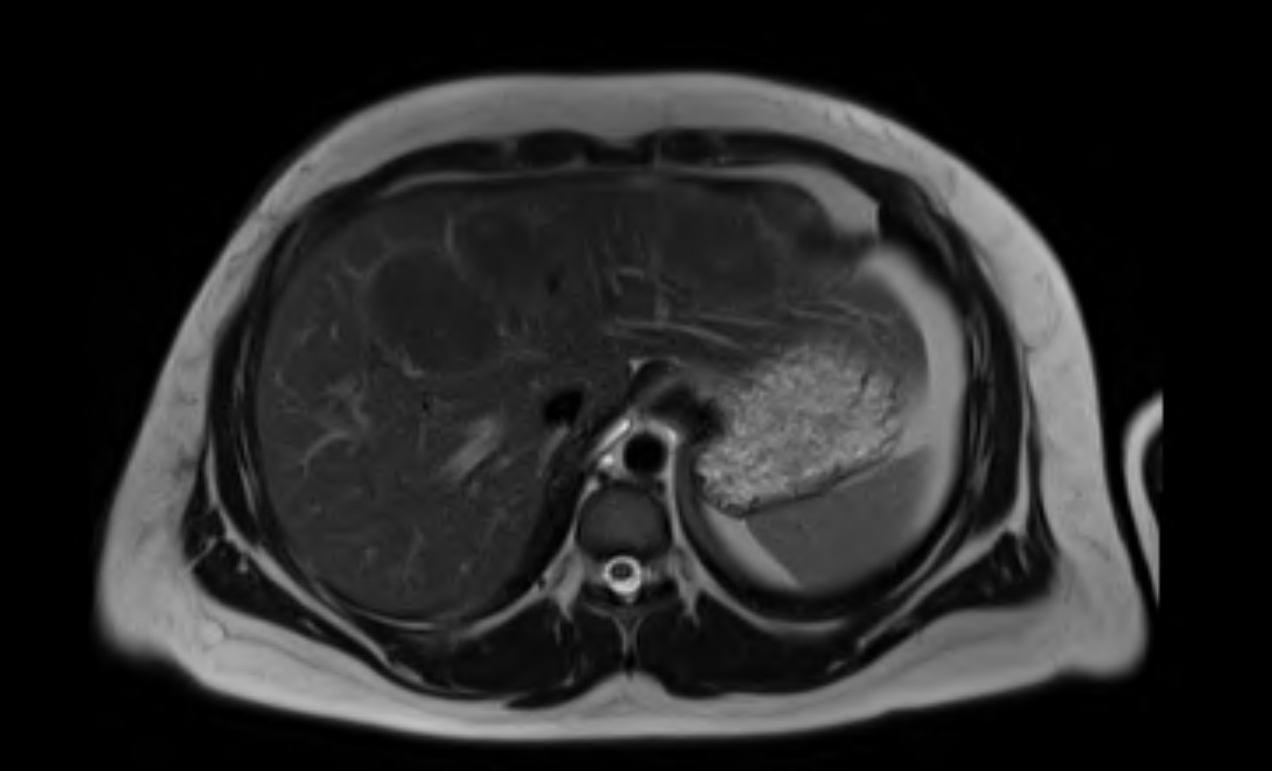
Figure: Figure 1. Axial T2-weighted MRI of the liver demonstrating two large hyperintense lesions, one in segment 8 (approximately 6 cm) and one in segment 4 (approximately 4 cm). The lesions appear well-circumscribed and markedly hyperintense relative to surrounding liver parenchyma.
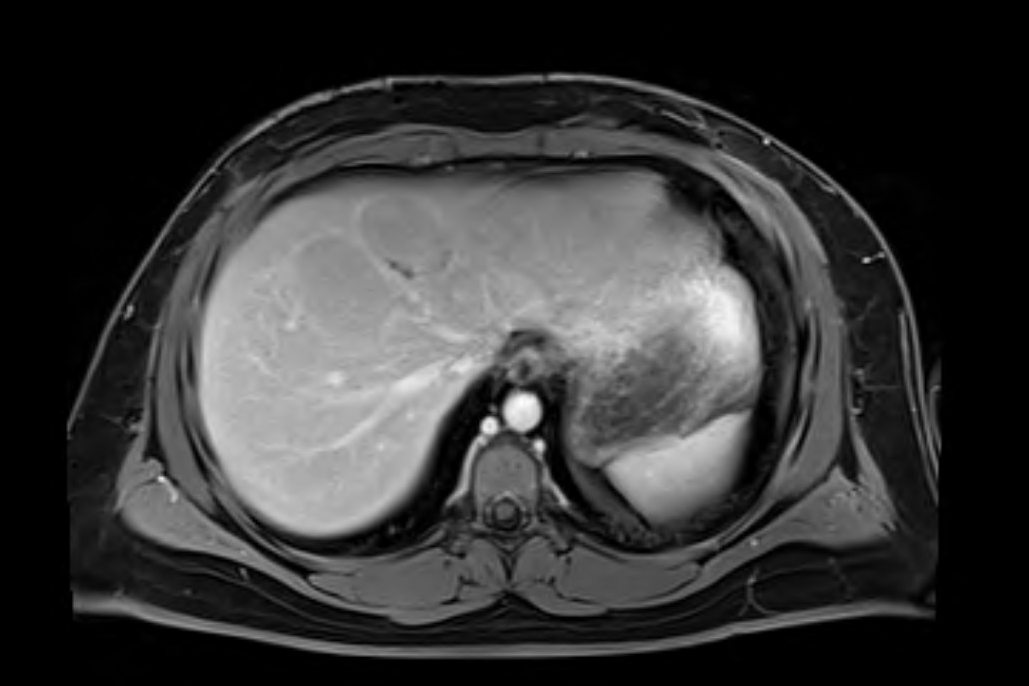
Figure: Figure 2. Axial T1-weighted MRI of the liver (pre-contrast) showing multiple hypointense lesions: the largest in segment 8 (6 cm) and another in segment 4 (4 cm). These masses appear well-demarcated and hypointense compared to the surrounding liver parenchyma.
Disclosures:
Thanh Nguyen indicated no relevant financial relationships.
Tzu-Hao Lee indicated no relevant financial relationships.
Thanh Nguyen, MD1, Tzu-Hao Lee, MD2. P6061 - Hepatocellular Adenoma Associated With Testosterone-Based Gender-Affirming Hormone Therapy in a Transgender Man, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
11. Department of Internal Medicine, Highland Hospital, Oakland, CA, USA, Oakland, CA; 22. Section of Gastroenterology and Hepatology, Department of Medicine, Baylor College of Medicine, Houston, TX, USA., Houston, TX
Introduction:
Introduction: Transgender and gender-diverse (TGD) individuals may receive gender-affirming hormone therapy (GAHT), testosterone for transgender men and estradiol for transgender women, to align their physical appearance with gender identity. While GAHT is generally safe, rare associations between long-term testosterone use and hepatobiliary neoplasms, particularly hepatocellular adenoma in transgender men, have been reported. We describe a case of hepatocellular adenoma in a transgender man likely linked to prolonged testosterone therapy.
Case Description/
Methods:
Case Presentation: A 19-year-old transgender man on transdermal testosterone and norethindrone for four years presented with chronic abdominal cramping. His medical history included dyslipidemia (treated with atorvastatin) and new persistent liver enzyme elevations over the preceding six months. He denied alcohol and tobacco use; family history was unremarkable. Physical examination revealed a BMI of 33 kg/m² with otherwise unremarkable exam. Laboratory studies showed elevated ALT (121 U/L), AST (44 U/L), and ALP (148 U/L); normal bilirubin (0.4 mg/dL), albumin (4.7 g/dL), platelets (363 × 10³/µL), PT/INR, and CRP; and a positive ANA (1:160) with negative anti–smooth muscle antibody and negative viral hepatitis serologies. An abdominal ultrasound performed 18 months prior had shown increased hepatic echogenicity without focal lesions. Current MRI identified three liver masses, the largest measuring 6 cm in segment 8. Percutaneous biopsy confirmed hepatocellular adenoma. Testosterone therapy was held, and plans were made for close follow-up with interval imaging.
Discussion:
Discussion: Hepatocellular adenomas in transgender men on testosterone-based GAHT are rare, with fewer than five cases reported to date, and progestins appear to play a negligible role in this context. Because uninterrupted access to GAHT is vital for many TGD individuals, a multidisciplinary approach is critical to enable prompt identification and management of hepatic lesions while minimizing interruptions to GAHT. Clinicians should remain vigilant for potential liver complications in TGD patients receiving testosterone, ensuring that hormone therapy can continue safely whenever feasible. Larger cohort studies and dedicated registries are needed to determine the true incidence and risk factors for hepatobiliary neoplasms in this population.

Figure: Figure 1. Axial T2-weighted MRI of the liver demonstrating two large hyperintense lesions, one in segment 8 (approximately 6 cm) and one in segment 4 (approximately 4 cm). The lesions appear well-circumscribed and markedly hyperintense relative to surrounding liver parenchyma.

Figure: Figure 2. Axial T1-weighted MRI of the liver (pre-contrast) showing multiple hypointense lesions: the largest in segment 8 (6 cm) and another in segment 4 (4 cm). These masses appear well-demarcated and hypointense compared to the surrounding liver parenchyma.
Disclosures:
Thanh Nguyen indicated no relevant financial relationships.
Tzu-Hao Lee indicated no relevant financial relationships.
Thanh Nguyen, MD1, Tzu-Hao Lee, MD2. P6061 - Hepatocellular Adenoma Associated With Testosterone-Based Gender-Affirming Hormone Therapy in a Transgender Man, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
