Tuesday Poster Session
Category: Liver
P6058 - Idiosyncratic Drug-Induced Liver Injury From Labetalol in a Pregnant Patient
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Natalie Rosseau, MD (she/her/hers)
University of Florida Health Shands Hospital
Gainesville, FL
Presenting Author(s)
Natalie Rosseau, MD1, Christopher Miquel-Chambers, MD2, Maria Oliver-Ricart, MD2, Lehar Khanna, MD2, Roberto Firpi-Morell, MD, MS, FACG2
1University of Florida Health Shands Hospital, Gainesville, FL; 2University of Florida College of Medicine, Gainesville, FL
Introduction: Drug-induced liver injury (DILI) during pregnancy can be a challenging diagnosis. Pre-existing liver disease increases the risk of developing DILI. We present the case of a 35-year-old pregnant patient who developed rare DILI secondary to labetalol, where prompt recognition and management resulted in complete recovery with positive outcome.
Case Description/
Methods: A 35-year-old patient with obesity, hepatic steatosis, and unprovoked deep vein thrombosis three years prior presented at 30 weeks of gestational age to the hospital with a one-day history of right-sided abdominal pain. Her medications included prenatal vitamins, progesterone vaginal cream, and a new prescription of labetalol 200mg BID for palpitations three months prior. The patient had stable vital signs with reassuring fetal tracings. Initial laboratory analysis revealed a new hepatocellular liver injury, with AST 303 IU/L, ALT 573 IU/L, and normal ALP and bilirubin levels. Tylenol, recreational drugs, and herbal and dietary supplements were ruled out. Ultrasound with Doppler showed hepatic steatosis without biliary disease. Extensive serological testing was negative for infections and autoimmune liver disease. The stable clinical presentation and overall unremarkable laboratory work-up did not support a pregnancy-associated liver disease diagnosis. Labetalol was discontinued due to suspicion of DILI one day after her presentation. The patient’s transaminases continued to worsen over the next 48 hours after labetalol discontinuation, prompting further investigations. MRCP showed moderate steatosis. Liver biopsy 4 days after her presentation revealed steatohepatitis with resolving injury suggestive of DILI. After 48 hours of labetalol discontinuation and day 4 of presentation, transaminases peaked. The patient was diagnosed with DILI from labetalol and discharged on day 6 with gradually improving transaminases. The patient’s liver functions completely normalized 5 weeks after labetalol discontinuation. The patient had an uncomplicated delivery at 37 weeks of gestation with normal liver function.
Discussion: DILI is an important differential diagnosis in pregnant patients with transaminitis, and a thorough work-up is necessary. Labetalol is a commonly used first-line medication for hypertension in pregnancy, with only a handful of cases of DILI from Labetalol in the literature. This case aims to highlight the importance of early recognition of DILI from labetalol use to prevent severe adverse outcomes.
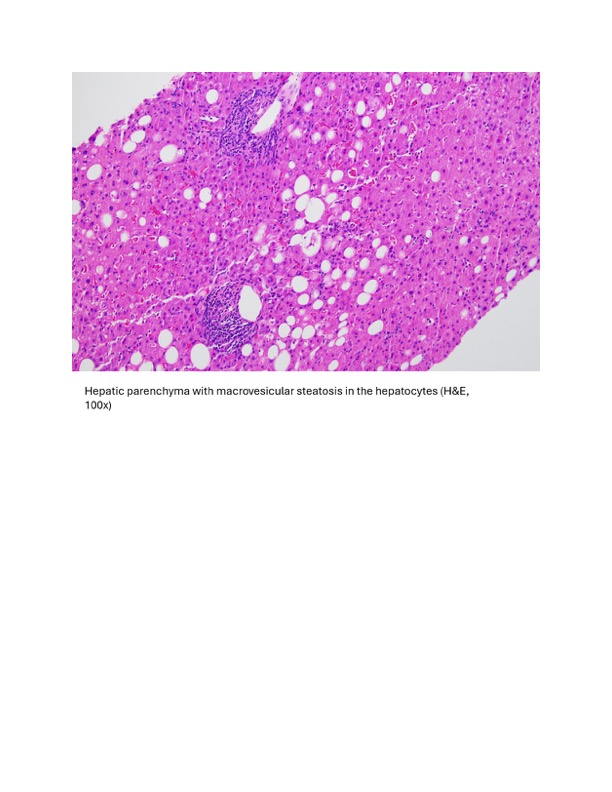
Figure: Histopathology Images
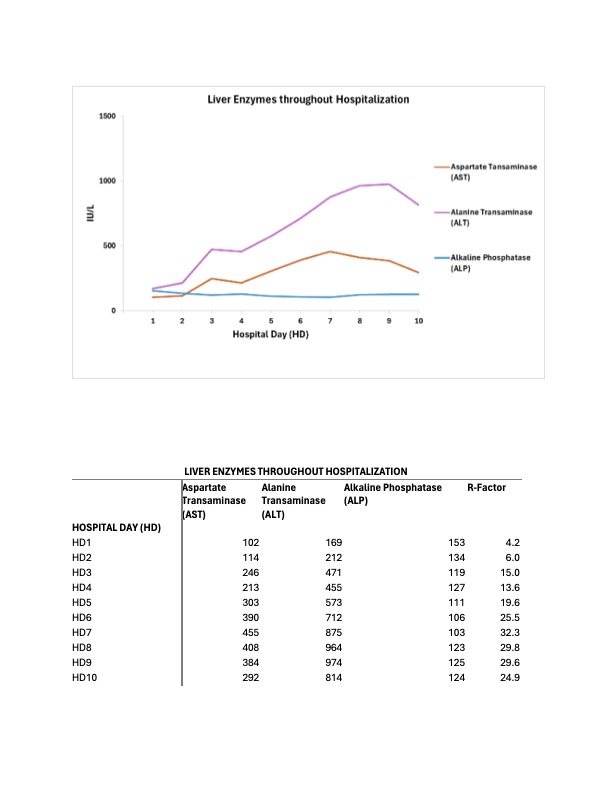
Figure: Liver Enzymes Graph and Table
Disclosures:
Natalie Rosseau indicated no relevant financial relationships.
Christopher Miquel-Chambers indicated no relevant financial relationships.
Maria Oliver-Ricart indicated no relevant financial relationships.
Lehar Khanna indicated no relevant financial relationships.
Roberto Firpi-Morell indicated no relevant financial relationships.
Natalie Rosseau, MD1, Christopher Miquel-Chambers, MD2, Maria Oliver-Ricart, MD2, Lehar Khanna, MD2, Roberto Firpi-Morell, MD, MS, FACG2. P6058 - Idiosyncratic Drug-Induced Liver Injury From Labetalol in a Pregnant Patient, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Florida Health Shands Hospital, Gainesville, FL; 2University of Florida College of Medicine, Gainesville, FL
Introduction: Drug-induced liver injury (DILI) during pregnancy can be a challenging diagnosis. Pre-existing liver disease increases the risk of developing DILI. We present the case of a 35-year-old pregnant patient who developed rare DILI secondary to labetalol, where prompt recognition and management resulted in complete recovery with positive outcome.
Case Description/
Methods: A 35-year-old patient with obesity, hepatic steatosis, and unprovoked deep vein thrombosis three years prior presented at 30 weeks of gestational age to the hospital with a one-day history of right-sided abdominal pain. Her medications included prenatal vitamins, progesterone vaginal cream, and a new prescription of labetalol 200mg BID for palpitations three months prior. The patient had stable vital signs with reassuring fetal tracings. Initial laboratory analysis revealed a new hepatocellular liver injury, with AST 303 IU/L, ALT 573 IU/L, and normal ALP and bilirubin levels. Tylenol, recreational drugs, and herbal and dietary supplements were ruled out. Ultrasound with Doppler showed hepatic steatosis without biliary disease. Extensive serological testing was negative for infections and autoimmune liver disease. The stable clinical presentation and overall unremarkable laboratory work-up did not support a pregnancy-associated liver disease diagnosis. Labetalol was discontinued due to suspicion of DILI one day after her presentation. The patient’s transaminases continued to worsen over the next 48 hours after labetalol discontinuation, prompting further investigations. MRCP showed moderate steatosis. Liver biopsy 4 days after her presentation revealed steatohepatitis with resolving injury suggestive of DILI. After 48 hours of labetalol discontinuation and day 4 of presentation, transaminases peaked. The patient was diagnosed with DILI from labetalol and discharged on day 6 with gradually improving transaminases. The patient’s liver functions completely normalized 5 weeks after labetalol discontinuation. The patient had an uncomplicated delivery at 37 weeks of gestation with normal liver function.
Discussion: DILI is an important differential diagnosis in pregnant patients with transaminitis, and a thorough work-up is necessary. Labetalol is a commonly used first-line medication for hypertension in pregnancy, with only a handful of cases of DILI from Labetalol in the literature. This case aims to highlight the importance of early recognition of DILI from labetalol use to prevent severe adverse outcomes.

Figure: Histopathology Images

Figure: Liver Enzymes Graph and Table
Disclosures:
Natalie Rosseau indicated no relevant financial relationships.
Christopher Miquel-Chambers indicated no relevant financial relationships.
Maria Oliver-Ricart indicated no relevant financial relationships.
Lehar Khanna indicated no relevant financial relationships.
Roberto Firpi-Morell indicated no relevant financial relationships.
Natalie Rosseau, MD1, Christopher Miquel-Chambers, MD2, Maria Oliver-Ricart, MD2, Lehar Khanna, MD2, Roberto Firpi-Morell, MD, MS, FACG2. P6058 - Idiosyncratic Drug-Induced Liver Injury From Labetalol in a Pregnant Patient, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
