Tuesday Poster Session
Category: Liver
P6145 - Severe Pembrolizumab-Induced Liver Injury: Subacute Hepatic Necrosis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Madison Drogy, BS (she/her/hers)
New York Medical College
Valhalla, NY
Presenting Author(s)
Madison Drogy, BS1, Makeda Dawkins, MD2, Cynthia Cohen, MD2, David C.. Wolf, MD, FACG2, Garrett Smith, MD, PhD2
1New York Medical College, Valhalla, NY; 2Westchester Medical Center, Valhalla, NY
Introduction: Immune checkpoint inhibitors (ICIs) are a cornerstone of oncologic therapy but may cause hepatotoxicity, with severity determined per Common Terminology Criteria for Adverse Events (CTCAE). Pembrolizumab, a programmed cell death monoclonal antibody, can cause mild-to-moderate self-limiting transaminitis. This case highlights the presentation, diagnosis and treatment of severe subacute pembrolizumab-induced hepatitis.
Case Description/
Methods: A 41-year-old female with a history of triple negative breast cancer treated with neoadjuvant therapy (doxorubicin and pembrolizumab, last infusion 2 months prior) and lumpectomy was referred to the hepatology department for severe asymptomatic hepatocellular transaminitis. Labs showed the following: aspartate aminotransferase (AST) 2010 U/L, alanine aminotransferase (ALT) 1978 U/L, alkaline phosphate 166 U/L, and total bilirubin 1.6 mg/dL. Physical exam was unremarkable. Cross sectional imaging showed nonspecific periportal edema with patent hepatoportal vasculature. Serologies for hepatitis A, B, C, and E were negative as were epstein-barr, cytomegalovirus, and herpes simplex virus. Autoimmune hepatitis antibodies were negative. Empiric treatment for CTCAE Grade 4 ICI hepatitiswas initiated with methylprednisone 2 mg/kg/day IV. Despite this, transaminases worsened (AST 2664 U/L, ALT 2629 U/L), and coagulopathy developed (INR 1.8). N-acetylcysteine was initiated however, discontinued due to hyperemesis. Liver biopsy histopathology showed portal inflammation with bridging necrosis and lymphocytic, eosinophilic, and neutrophilic infiltration (Figure 1). Mycophenolate mofetil (MMF) was initiated after three days of steroid therapy due to inadequate biochemical response. Her transaminases gradually improved and MMF was tapered.
Discussion: Although checkpoint inhibitors typically cause indirect immune-mediated hepatotoxicity within 8-12 weeks of treatment, late-onset sequelae can be seen months after treatment. Pembrolizumab is known to cause transaminitis in 20-30% of patients undergoing active therapy, however is usually self-limiting, resolving even with continued administration. Only 1-2% of patients develop severe immune-mediated liver injury, often seen in cases of combination antineoplastic therapy. This case demonstrates the potential severity of subacute ICI hepatotoxicity following treatment completion, educating clinicians to consider ICI hepatitis in patients presenting with transaminitis and with a history of immune checkpoint inhibitor use.
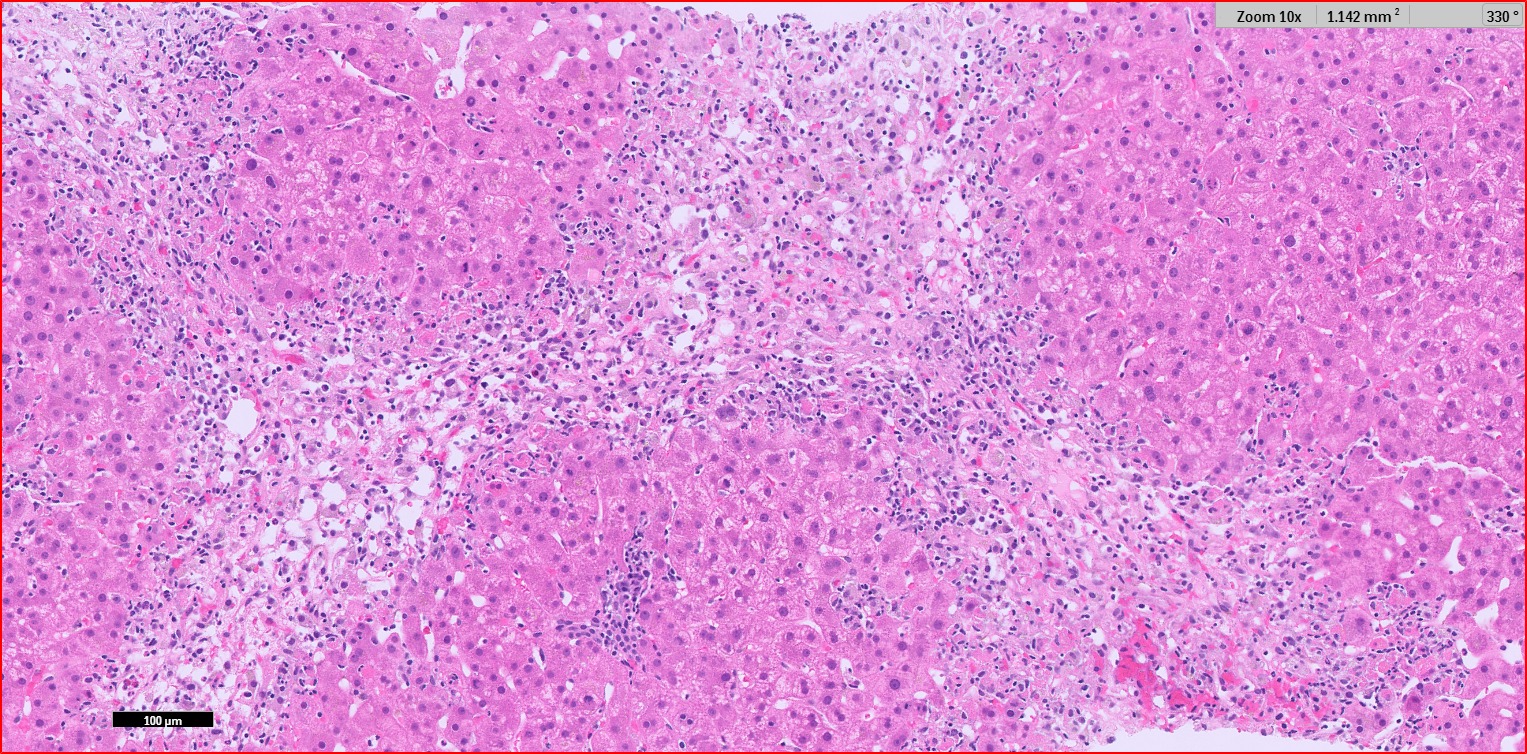
Figure: Histopathology of liver biopsy. Hematoxylin-eosin staining showing portal inflammation with bridging necrosis and lymphocytic, eosinophilic, and neutrophilic infiltration.
Disclosures:
Madison Drogy indicated no relevant financial relationships.
Makeda Dawkins indicated no relevant financial relationships.
Cynthia Cohen indicated no relevant financial relationships.
David Wolf indicated no relevant financial relationships.
Garrett Smith indicated no relevant financial relationships.
Madison Drogy, BS1, Makeda Dawkins, MD2, Cynthia Cohen, MD2, David C.. Wolf, MD, FACG2, Garrett Smith, MD, PhD2. P6145 - Severe Pembrolizumab-Induced Liver Injury: Subacute Hepatic Necrosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1New York Medical College, Valhalla, NY; 2Westchester Medical Center, Valhalla, NY
Introduction: Immune checkpoint inhibitors (ICIs) are a cornerstone of oncologic therapy but may cause hepatotoxicity, with severity determined per Common Terminology Criteria for Adverse Events (CTCAE). Pembrolizumab, a programmed cell death monoclonal antibody, can cause mild-to-moderate self-limiting transaminitis. This case highlights the presentation, diagnosis and treatment of severe subacute pembrolizumab-induced hepatitis.
Case Description/
Methods: A 41-year-old female with a history of triple negative breast cancer treated with neoadjuvant therapy (doxorubicin and pembrolizumab, last infusion 2 months prior) and lumpectomy was referred to the hepatology department for severe asymptomatic hepatocellular transaminitis. Labs showed the following: aspartate aminotransferase (AST) 2010 U/L, alanine aminotransferase (ALT) 1978 U/L, alkaline phosphate 166 U/L, and total bilirubin 1.6 mg/dL. Physical exam was unremarkable. Cross sectional imaging showed nonspecific periportal edema with patent hepatoportal vasculature. Serologies for hepatitis A, B, C, and E were negative as were epstein-barr, cytomegalovirus, and herpes simplex virus. Autoimmune hepatitis antibodies were negative. Empiric treatment for CTCAE Grade 4 ICI hepatitiswas initiated with methylprednisone 2 mg/kg/day IV. Despite this, transaminases worsened (AST 2664 U/L, ALT 2629 U/L), and coagulopathy developed (INR 1.8). N-acetylcysteine was initiated however, discontinued due to hyperemesis. Liver biopsy histopathology showed portal inflammation with bridging necrosis and lymphocytic, eosinophilic, and neutrophilic infiltration (Figure 1). Mycophenolate mofetil (MMF) was initiated after three days of steroid therapy due to inadequate biochemical response. Her transaminases gradually improved and MMF was tapered.
Discussion: Although checkpoint inhibitors typically cause indirect immune-mediated hepatotoxicity within 8-12 weeks of treatment, late-onset sequelae can be seen months after treatment. Pembrolizumab is known to cause transaminitis in 20-30% of patients undergoing active therapy, however is usually self-limiting, resolving even with continued administration. Only 1-2% of patients develop severe immune-mediated liver injury, often seen in cases of combination antineoplastic therapy. This case demonstrates the potential severity of subacute ICI hepatotoxicity following treatment completion, educating clinicians to consider ICI hepatitis in patients presenting with transaminitis and with a history of immune checkpoint inhibitor use.

Figure: Histopathology of liver biopsy. Hematoxylin-eosin staining showing portal inflammation with bridging necrosis and lymphocytic, eosinophilic, and neutrophilic infiltration.
Disclosures:
Madison Drogy indicated no relevant financial relationships.
Makeda Dawkins indicated no relevant financial relationships.
Cynthia Cohen indicated no relevant financial relationships.
David Wolf indicated no relevant financial relationships.
Garrett Smith indicated no relevant financial relationships.
Madison Drogy, BS1, Makeda Dawkins, MD2, Cynthia Cohen, MD2, David C.. Wolf, MD, FACG2, Garrett Smith, MD, PhD2. P6145 - Severe Pembrolizumab-Induced Liver Injury: Subacute Hepatic Necrosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
