Tuesday Poster Session
Category: Small Intestine
P6235 - Olmesartan-Induced Enteropathy in an Older Patient with Chronic Diarrhea
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- KK
Kruthi Kella, MD (she/her/hers)
Valley Health System, Icahn School of Medicine at Mount Sinai
Paramus, NJ
Presenting Author(s)
Kruthi Kella, MD, Amy Yeung, DO, Jonathan Pinto, MD, Michael Herman, MD, Shereen Santee, DO, Sajidur Rahman-Kader, MD, Metin Taskin, MD, Sita Chokhavatia, MD, MACG
Valley Health System, Icahn School of Medicine at Mount Sinai, Paramus, NJ
Introduction: Olmesartan is a commonly prescribed angiotensin II receptor blocker (ARB) for the treatment of hypertension. Although generally well-tolerated, it has been associated with a rare adverse effect such as sprue-like enteropathy characterized by chronic diarrhea and small bowel villous atrophy.
Case Description/
Methods: A 70-year-old woman with hypertension treated with a combination of amlodipine-olmesartan for 8 months presented with four weeks of nausea, vomiting, watery diarrhea and poor oral intake. She denied recent antibiotic use, travel, abdominal pain, melena, or fever. Prior colonoscopy and esophagogastroduodenoscopy (EGD) were normal.
Vitals and physical exam were unremarkable. Laboratory studies were normal, except for markedly elevated fecal calprotectin ( >1000 µg/g) and positive fecal occult blood test; stool cultures, Clostridium difficile toxin, and celiac serologies were negative. Chromogranin A levels were elevated but attributed to known proton pump inhibitor treatment. Abdominal contrast tomography (CT) and magnetic resonance imaging (MRI) showed no masses, and MRI enterography showed no features of Crohn’s disease.
Duodenal biopsies were obtained on a repeat grossly normal EGD. Two diminutive tubular adenoma polyps were resected at colonoscopy. Due to patient’s ongoing symptoms, capsule endoscopy was performed, revealing villous blunting in the small intestines (Figure 1), confirmed with duodenal biopsies (Figure 2). Based on clinical presentation and exclusion of infectious, inflammatory, and autoimmune causes, olmesartan-induced enteropathy was suspected. The drug was discontinued, and the patient was initiated on a short course of prednisone, resulting in symptom improvement.
Discussion: Olmesartan-induced enteropathy is a rare but increasingly recognized cause of chronic diarrhea, often occurring months to years after drug initiation. Elevated inflammatory markers such as fecal calprotectin or chromogranin A may reflect underlying inflammation but are not disease specific. Diagnosis relies on clinical suspicion, exclusion of other causes, and supportive findings on capsule endoscopy and biopsies. Among ARBs, olmesartan is the most strongly associated with this syndrome. This case illustrates the importance of reviewing medication history in patients with unexplained diarrhea in the absence of infectious or autoimmune etiologies. Early recognition, withdrawal of drug, and alternative hypertension treatment are crucial for symptom resolution.
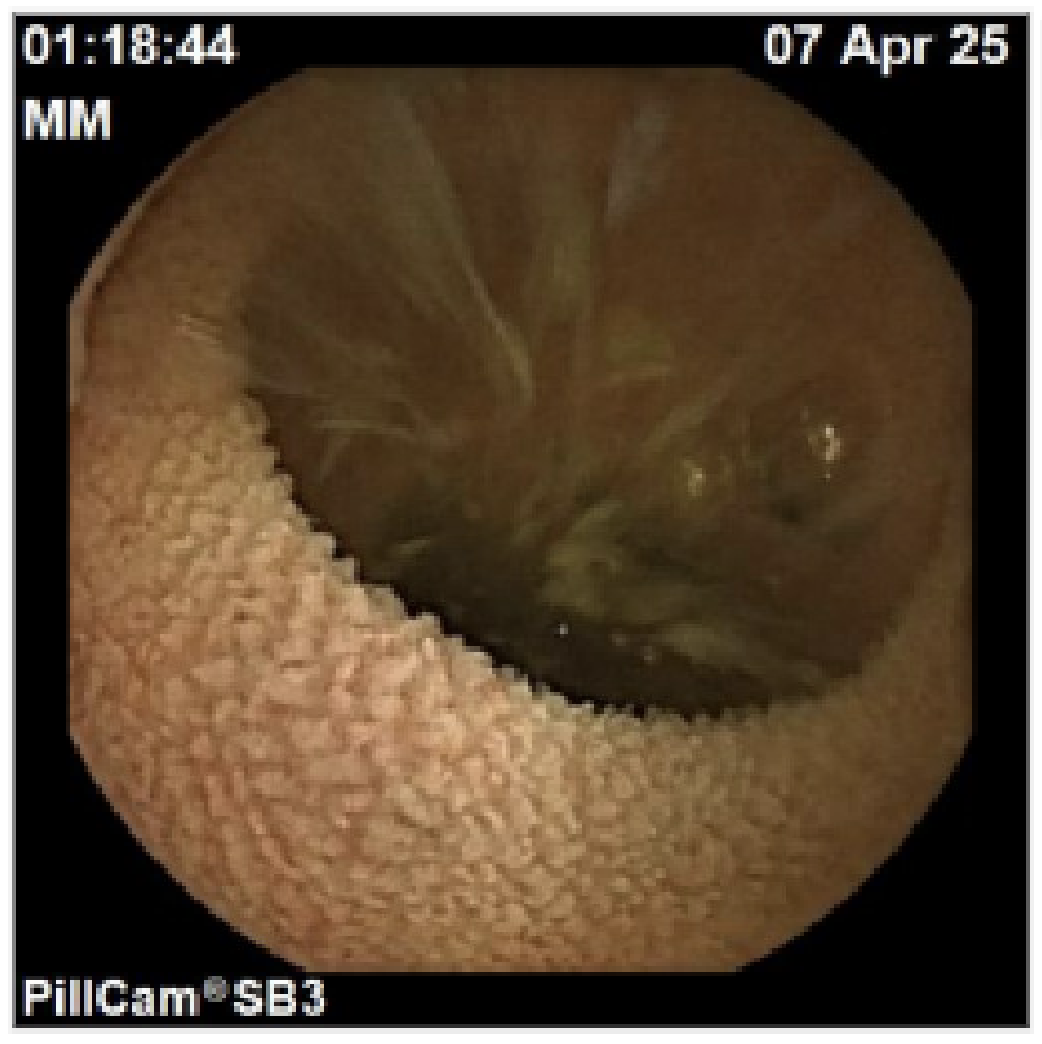
Figure: Figure 1: Capsule endoscopy of small bowel with villous blunting.

Figure: Figure 2: 100x HE. Duodenum with villus blunting and inflammation
Disclosures:
Kruthi Kella indicated no relevant financial relationships.
Amy Yeung indicated no relevant financial relationships.
Jonathan Pinto indicated no relevant financial relationships.
Michael Herman indicated no relevant financial relationships.
Shereen Santee indicated no relevant financial relationships.
Sajidur Rahman-Kader indicated no relevant financial relationships.
Metin Taskin indicated no relevant financial relationships.
Sita Chokhavatia indicated no relevant financial relationships.
Kruthi Kella, MD, Amy Yeung, DO, Jonathan Pinto, MD, Michael Herman, MD, Shereen Santee, DO, Sajidur Rahman-Kader, MD, Metin Taskin, MD, Sita Chokhavatia, MD, MACG. P6235 - Olmesartan-Induced Enteropathy in an Older Patient with Chronic Diarrhea, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Valley Health System, Icahn School of Medicine at Mount Sinai, Paramus, NJ
Introduction: Olmesartan is a commonly prescribed angiotensin II receptor blocker (ARB) for the treatment of hypertension. Although generally well-tolerated, it has been associated with a rare adverse effect such as sprue-like enteropathy characterized by chronic diarrhea and small bowel villous atrophy.
Case Description/
Methods: A 70-year-old woman with hypertension treated with a combination of amlodipine-olmesartan for 8 months presented with four weeks of nausea, vomiting, watery diarrhea and poor oral intake. She denied recent antibiotic use, travel, abdominal pain, melena, or fever. Prior colonoscopy and esophagogastroduodenoscopy (EGD) were normal.
Vitals and physical exam were unremarkable. Laboratory studies were normal, except for markedly elevated fecal calprotectin ( >1000 µg/g) and positive fecal occult blood test; stool cultures, Clostridium difficile toxin, and celiac serologies were negative. Chromogranin A levels were elevated but attributed to known proton pump inhibitor treatment. Abdominal contrast tomography (CT) and magnetic resonance imaging (MRI) showed no masses, and MRI enterography showed no features of Crohn’s disease.
Duodenal biopsies were obtained on a repeat grossly normal EGD. Two diminutive tubular adenoma polyps were resected at colonoscopy. Due to patient’s ongoing symptoms, capsule endoscopy was performed, revealing villous blunting in the small intestines (Figure 1), confirmed with duodenal biopsies (Figure 2). Based on clinical presentation and exclusion of infectious, inflammatory, and autoimmune causes, olmesartan-induced enteropathy was suspected. The drug was discontinued, and the patient was initiated on a short course of prednisone, resulting in symptom improvement.
Discussion: Olmesartan-induced enteropathy is a rare but increasingly recognized cause of chronic diarrhea, often occurring months to years after drug initiation. Elevated inflammatory markers such as fecal calprotectin or chromogranin A may reflect underlying inflammation but are not disease specific. Diagnosis relies on clinical suspicion, exclusion of other causes, and supportive findings on capsule endoscopy and biopsies. Among ARBs, olmesartan is the most strongly associated with this syndrome. This case illustrates the importance of reviewing medication history in patients with unexplained diarrhea in the absence of infectious or autoimmune etiologies. Early recognition, withdrawal of drug, and alternative hypertension treatment are crucial for symptom resolution.

Figure: Figure 1: Capsule endoscopy of small bowel with villous blunting.

Figure: Figure 2: 100x HE. Duodenum with villus blunting and inflammation
Disclosures:
Kruthi Kella indicated no relevant financial relationships.
Amy Yeung indicated no relevant financial relationships.
Jonathan Pinto indicated no relevant financial relationships.
Michael Herman indicated no relevant financial relationships.
Shereen Santee indicated no relevant financial relationships.
Sajidur Rahman-Kader indicated no relevant financial relationships.
Metin Taskin indicated no relevant financial relationships.
Sita Chokhavatia indicated no relevant financial relationships.
Kruthi Kella, MD, Amy Yeung, DO, Jonathan Pinto, MD, Michael Herman, MD, Shereen Santee, DO, Sajidur Rahman-Kader, MD, Metin Taskin, MD, Sita Chokhavatia, MD, MACG. P6235 - Olmesartan-Induced Enteropathy in an Older Patient with Chronic Diarrhea, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
