Monday Poster Session
Category: Biliary/Pancreas
P2281 - Case Report: Beware of Incomplete Workup: A Case of Acute Pancreatitis Due to Pancreatic Malignancy Misdiagnosed as GLP-1 Receptor Agonist Side Effects
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Khadeja Khan, MD (she/her/hers)
University of Miami Miller School of Medicine at Jackson Memorial Hospital
Miami, FL
Presenting Author(s)
Khadeja Khan, MD1, Jodie A. Barkin, MD, FACG2, Sean Bhalla, MD2
1University of Miami Miller School of Medicine at Jackson Memorial Hospital, Miami, FL; 2University of Miami Miller School of Medicine, Miami, FL
Introduction: Glucagon-Like Peptide-1 Receptor Agonist (GLP-1RA) use for treatment of type 2 diabetes mellitus (T2DM) and weight loss is rising. GLP-1RAs have multiple known GI side effects such as abdominal pain, nausea, vomiting, early satiety, elevation in pancreatic enzymes and acute pancreatitis (AP). Unfortunately these side effects may also mask vague symptoms of pancreatic malignancy. We present a case of AP attributed to GLP-1RA use, which led to delayed diagnosis of pancreatic adenocarcinoma.
Case Description/
Methods: A 62y/o woman with obesity, chronic kidney disease (CKD), and T2DM presented to the ED with 1 week of worsening intermittent LUQ abdominal pain and limited oral intake. Two months prior, she started Semaglutide for weight loss. Labs were notable for elevated lipase (1,332) and creatinine (1.35). CT abdomen pelvis without contrast due to CKD showed new thickening and stranding around the body of the pancreas (Fig 1A). A diagnosis of AP was made. She was admitted and managed conservatively. Workup was negative for alcohol, tobacco, or labs (normal LFTs, triglycerides, IgG4). AP was attributed to GLP1-RA, and GLP-1RA was stopped.
At subsequent GI evaluation 4 months later, she was asymptomatic, but workup was pursued given the lack of prior follow-up or contrast-enhanced imaging. Due to CKD and contrast limitations, EUS was performed instead, revealing a 44x43mm hypoechoic mass in the pancreatic body with parenchymal changes (Fig 2). Repeat CT with contrast confirmed the mass (Fig 1B). EUS-guided biopsy showed moderately to poorly differentiated pancreatic ductal adenocarcinoma. She began neoadjuvant gemcitabine/Abraxane nearly 8 months after initial symptoms, followed by Whipple surgery (pT3N0) with poor prognosis.
Discussion: GLP-1RAs are considered safe, with AP noted as a rare but potential side effect. However, their GI profile may mask or delay diagnosis of pancreatic malignancy given the vague nature of symptoms—abdominal pain, weight loss, early satiety, and AP—all of which may be misattributed to GLP-1RA use. Anchoring bias and premature closure may lead clinicians to overlook red flags.
This case highlights the risk of attributing AP to GLP-1RA therapy without thorough evaluation of alternative causes, particularly in the absence of confirmatory follow-up contrast-enhanced imaging and delaying cancer diagnosis by several months. Detailed workup of AP etiologies is crucial to prevent morbidity and mortality from delayed diagnosis of underlying pancreatic malignancy.
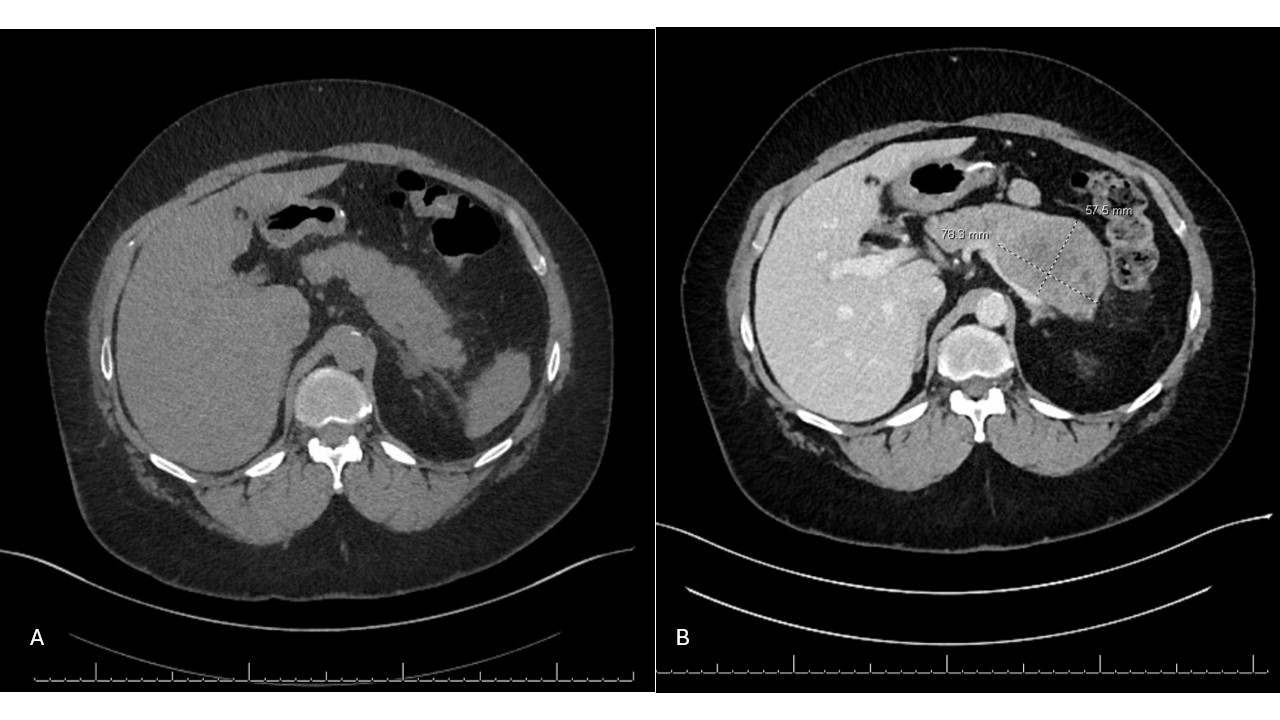
Figure: Figure 1. A: CT Abdomen w/o contrast on first presentation 8/11/23 for acute pancreatitis. B: Repeat CTAP with contrast 10 months after initial presentation showed large pancreatic adenocarcinoma, 78.9 mm in largest dimension

Figure: Figure 2. EUS of the pancreas showing a 44 x 43 mm mass in the pancreatic body.
Disclosures:
Khadeja Khan indicated no relevant financial relationships.
Jodie Barkin: AbbVie – Advisor or Review Panel Member. Aimmune Therapeutics – Advisor or Review Panel Member. Amgen – Consultant. CorEvitas – Consultant. Iterative Health – Consultant. Medtronic – Consultant. MotusGI – Consultant.
Sean Bhalla indicated no relevant financial relationships.
Khadeja Khan, MD1, Jodie A. Barkin, MD, FACG2, Sean Bhalla, MD2. P2281 -
Case Report: Beware of Incomplete Workup: A Case of Acute Pancreatitis Due to Pancreatic Malignancy Misdiagnosed as GLP-1 Receptor Agonist Side Effects, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Miami Miller School of Medicine at Jackson Memorial Hospital, Miami, FL; 2University of Miami Miller School of Medicine, Miami, FL
Introduction: Glucagon-Like Peptide-1 Receptor Agonist (GLP-1RA) use for treatment of type 2 diabetes mellitus (T2DM) and weight loss is rising. GLP-1RAs have multiple known GI side effects such as abdominal pain, nausea, vomiting, early satiety, elevation in pancreatic enzymes and acute pancreatitis (AP). Unfortunately these side effects may also mask vague symptoms of pancreatic malignancy. We present a case of AP attributed to GLP-1RA use, which led to delayed diagnosis of pancreatic adenocarcinoma.
Case Description/
Methods: A 62y/o woman with obesity, chronic kidney disease (CKD), and T2DM presented to the ED with 1 week of worsening intermittent LUQ abdominal pain and limited oral intake. Two months prior, she started Semaglutide for weight loss. Labs were notable for elevated lipase (1,332) and creatinine (1.35). CT abdomen pelvis without contrast due to CKD showed new thickening and stranding around the body of the pancreas (Fig 1A). A diagnosis of AP was made. She was admitted and managed conservatively. Workup was negative for alcohol, tobacco, or labs (normal LFTs, triglycerides, IgG4). AP was attributed to GLP1-RA, and GLP-1RA was stopped.
At subsequent GI evaluation 4 months later, she was asymptomatic, but workup was pursued given the lack of prior follow-up or contrast-enhanced imaging. Due to CKD and contrast limitations, EUS was performed instead, revealing a 44x43mm hypoechoic mass in the pancreatic body with parenchymal changes (Fig 2). Repeat CT with contrast confirmed the mass (Fig 1B). EUS-guided biopsy showed moderately to poorly differentiated pancreatic ductal adenocarcinoma. She began neoadjuvant gemcitabine/Abraxane nearly 8 months after initial symptoms, followed by Whipple surgery (pT3N0) with poor prognosis.
Discussion: GLP-1RAs are considered safe, with AP noted as a rare but potential side effect. However, their GI profile may mask or delay diagnosis of pancreatic malignancy given the vague nature of symptoms—abdominal pain, weight loss, early satiety, and AP—all of which may be misattributed to GLP-1RA use. Anchoring bias and premature closure may lead clinicians to overlook red flags.
This case highlights the risk of attributing AP to GLP-1RA therapy without thorough evaluation of alternative causes, particularly in the absence of confirmatory follow-up contrast-enhanced imaging and delaying cancer diagnosis by several months. Detailed workup of AP etiologies is crucial to prevent morbidity and mortality from delayed diagnosis of underlying pancreatic malignancy.

Figure: Figure 1. A: CT Abdomen w/o contrast on first presentation 8/11/23 for acute pancreatitis. B: Repeat CTAP with contrast 10 months after initial presentation showed large pancreatic adenocarcinoma, 78.9 mm in largest dimension

Figure: Figure 2. EUS of the pancreas showing a 44 x 43 mm mass in the pancreatic body.
Disclosures:
Khadeja Khan indicated no relevant financial relationships.
Jodie Barkin: AbbVie – Advisor or Review Panel Member. Aimmune Therapeutics – Advisor or Review Panel Member. Amgen – Consultant. CorEvitas – Consultant. Iterative Health – Consultant. Medtronic – Consultant. MotusGI – Consultant.
Sean Bhalla indicated no relevant financial relationships.
Khadeja Khan, MD1, Jodie A. Barkin, MD, FACG2, Sean Bhalla, MD2. P2281 -
Case Report: Beware of Incomplete Workup: A Case of Acute Pancreatitis Due to Pancreatic Malignancy Misdiagnosed as GLP-1 Receptor Agonist Side Effects, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
