Monday Poster Session
Category: Colon
P2397 - Survival Outcomes in Immune-Mediated Diarrhea and Colitis Patients Treated With Fecal Microbiota Transplantation and Selective Immunosuppressive Therapy
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- CC
Carolina Colli Cruz, MD
University of Texas MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Carolina Cruz, MD1, Maria Julia M. N.. Santos, MD2, Sharada Wali, MBBS, MPH1, Krishnavathana Varatharajalu, MD2, Yinghong Wang, MD, PhD, MS1
1University of Texas MD Anderson Cancer Center, Houston, TX; 2MD Anderson Cancer Center, Houston, TX
Introduction: Response rates to immune checkpoint inhibitors (ICI) vary widely—15–30% in most solid tumors and 45–60% in melanoma. Fecal microbiota transplantation (FMT) from ICI responders is under investigation to improve outcomes in non-responders by modulating their microbiome. Additionally, FMT from healthy donors has been used to treat immune-mediated diarrhea and colitis (IMDC). This study evaluates whether FMT from healthy donors can influence cancer progression or survival in IMDC patients compared to standard selective immunosuppressive therapy (SIT) alone.
Methods: This retrospective single-center study included IMDC patients enrolled in NCT04407247 (Group 1:corticosteroids + SIT) and NCT03819296 (Group 2: corticosteroids + SIT + FMT). Statistical analysis was performed using SPSS v24.
Results: 98 patients were included: 42 in Group 1 and 54 in Group 2. Baseline characteristics and intergroup comparisons are detailed in Tables 1 and 2. Multivariate logistic regression showed that a higher number of ICI doses prior to IMDC was protective against cancer progression (OR: 0.9, p=0.047), with a trend favoring PD-1/PD-L1 therapy (OR: 0.05, p=0.099). Initiation of new chemotherapy within six months of toxicity onset was associated with cancer progression (OR: 11.9, p=0.001). Cox multivariate analysis identified increased mortality linked to resumption of initial chemotherapy post-IMDC (HR: 28.4, p=0.013) and initial infliximab use (HR: 22.1, p=0.033) for IMDC. Higher number of SIT doses were significantly associated with improved survival (HR: 0.2, p=0.040). Kaplan-Meier analysis indicated a trend toward longer survival in the SIT + FMT group versus SIT alone (mean 60.2 vs 28.7 months; p=0.097). Vedolizumab was associated with significantly longer survival (mean 55.0 months) compared to infliximab (mean 26.7 months; p=0.016).
Discussion: This study suggests that treatments e.g. SIT and FMT could improve the IMDC condition, but do not promote cancer progression. Vedolizumab was independently associated with better survival than infliximab. Adding FMT to SIT may improve survival further in IMDC patients. These findings warrant further research.
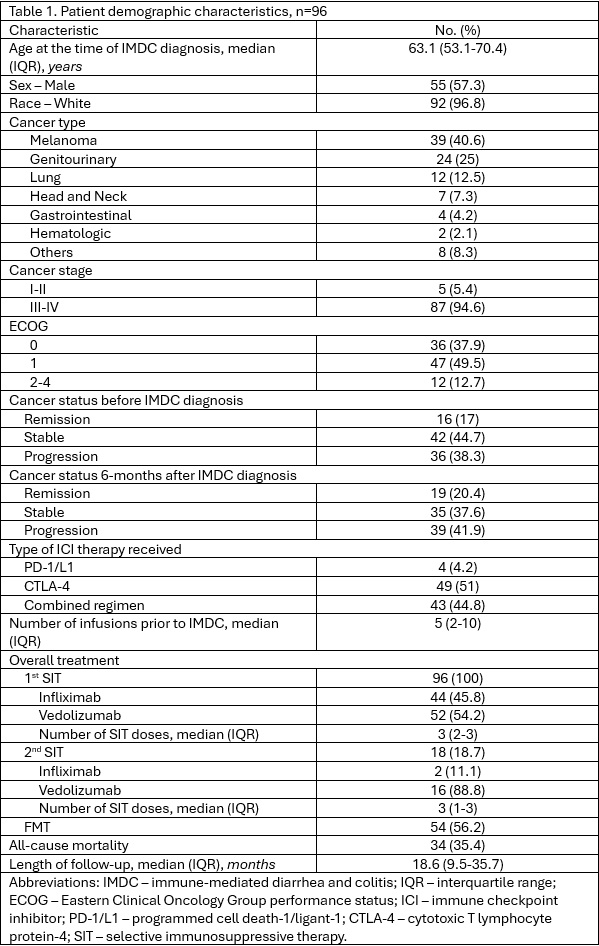
Figure: Table 1.
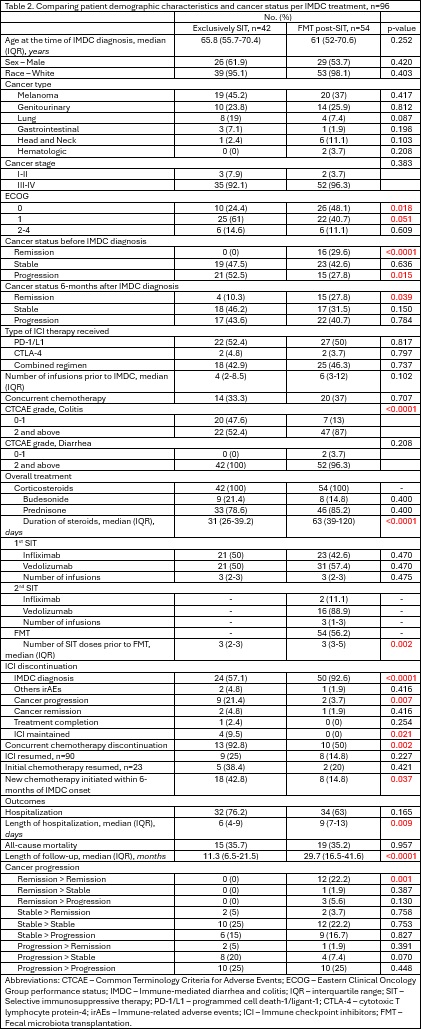
Figure: Table 2.
Disclosures:
Carolina Cruz indicated no relevant financial relationships.
Maria Julia Santos indicated no relevant financial relationships.
Sharada Wali indicated no relevant financial relationships.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Carolina Cruz, MD1, Maria Julia M. N.. Santos, MD2, Sharada Wali, MBBS, MPH1, Krishnavathana Varatharajalu, MD2, Yinghong Wang, MD, PhD, MS1. P2397 - Survival Outcomes in Immune-Mediated Diarrhea and Colitis Patients Treated With Fecal Microbiota Transplantation and Selective Immunosuppressive Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Texas MD Anderson Cancer Center, Houston, TX; 2MD Anderson Cancer Center, Houston, TX
Introduction: Response rates to immune checkpoint inhibitors (ICI) vary widely—15–30% in most solid tumors and 45–60% in melanoma. Fecal microbiota transplantation (FMT) from ICI responders is under investigation to improve outcomes in non-responders by modulating their microbiome. Additionally, FMT from healthy donors has been used to treat immune-mediated diarrhea and colitis (IMDC). This study evaluates whether FMT from healthy donors can influence cancer progression or survival in IMDC patients compared to standard selective immunosuppressive therapy (SIT) alone.
Methods: This retrospective single-center study included IMDC patients enrolled in NCT04407247 (Group 1:corticosteroids + SIT) and NCT03819296 (Group 2: corticosteroids + SIT + FMT). Statistical analysis was performed using SPSS v24.
Results: 98 patients were included: 42 in Group 1 and 54 in Group 2. Baseline characteristics and intergroup comparisons are detailed in Tables 1 and 2. Multivariate logistic regression showed that a higher number of ICI doses prior to IMDC was protective against cancer progression (OR: 0.9, p=0.047), with a trend favoring PD-1/PD-L1 therapy (OR: 0.05, p=0.099). Initiation of new chemotherapy within six months of toxicity onset was associated with cancer progression (OR: 11.9, p=0.001). Cox multivariate analysis identified increased mortality linked to resumption of initial chemotherapy post-IMDC (HR: 28.4, p=0.013) and initial infliximab use (HR: 22.1, p=0.033) for IMDC. Higher number of SIT doses were significantly associated with improved survival (HR: 0.2, p=0.040). Kaplan-Meier analysis indicated a trend toward longer survival in the SIT + FMT group versus SIT alone (mean 60.2 vs 28.7 months; p=0.097). Vedolizumab was associated with significantly longer survival (mean 55.0 months) compared to infliximab (mean 26.7 months; p=0.016).
Discussion: This study suggests that treatments e.g. SIT and FMT could improve the IMDC condition, but do not promote cancer progression. Vedolizumab was independently associated with better survival than infliximab. Adding FMT to SIT may improve survival further in IMDC patients. These findings warrant further research.

Figure: Table 1.

Figure: Table 2.
Disclosures:
Carolina Cruz indicated no relevant financial relationships.
Maria Julia Santos indicated no relevant financial relationships.
Sharada Wali indicated no relevant financial relationships.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Carolina Cruz, MD1, Maria Julia M. N.. Santos, MD2, Sharada Wali, MBBS, MPH1, Krishnavathana Varatharajalu, MD2, Yinghong Wang, MD, PhD, MS1. P2397 - Survival Outcomes in Immune-Mediated Diarrhea and Colitis Patients Treated With Fecal Microbiota Transplantation and Selective Immunosuppressive Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
