Monday Poster Session
Category: Esophagus
P2735 - Endoscopic Ultrasound-Guided Injection of NBTXR3 Activated by Radiotherapy With Concurrent Chemotherapy for Esophageal Adenocarcinoma: Interim Analysis of Feasibility and Safety
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- EC
Emmanuel Coronel, MD
The University of Texas MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Emmanuel Coronel, MD1, George Wahba, MD2, Phillip S.. Ge, MD1, Saumil Gandhi, MD, PhD2, Juliana Bronk, MD, PhD2, David Qian, MD, PhD2, Joe Chang, MD, PhD2, Michael S. O'Reilly, MD2, Matthew Ning, MD, MPH2, Aileen B. Chen, MD2, Quynh-Nhu Nguyen, MD2, Diana Amaya, BSN2, Christina Hoang, BSN2, Wayne Hofstetter, MD2, Mariela Blum Murphy, MD2, Albert C.. Koong, MD, PhD2, Zhongxing Liao, MD, PhD2, Steven Lin, MD, PhD2
1The University of Texas MD Anderson Cancer Center, Houston, TX; 2MD Anderson Cancer Center, Houston, TX
Introduction: Despite definitive chemoradiation (CRT) being the standard of care for inoperable esophageal adenocarcinoma, only 25% of patients achieve a complete treatment response. This highlights the necessity to enhance cure rates for inoperable patients and to improve clinical outcomes for operable individuals. We report the interim results of a phase I trial of endoscopic ultrasound-guided fine needle tumor injection (EUS-FNI) of NBTXR3 (R3), a hafnium oxide nanoparticle that enhances the local radiation effect by 9-fold when activated by ionizing radiation.
Methods: This is a planned interim analysis of the feasibility and safety of the first 9 patients who underwent EUS-FNI of R3 (Figure 1) and received photon therapy. Patients with bulky mediastinal tumors or tumors above the carina are excluded. Patients received escalating doses of R3, corresponding to 15%, 22%, and 33% of the gross tumor volume (GTV). Investigational drug delivery of R3 was performed under EUS guidance; the tumor was injected at multiple sites to ensure uniform distribution within the mass. A 22-gauge needle was used for EUS-FNI, and the rate of injection was 1 cc/min. Verification CT scan is taken within 3 days of the injection to visualize the distribution of the radiopaque R3 prior to starting CRT.
Results: Only one patient each was treated at 15% and 22%, with 7 treated at 33% of the GTV, a total of 9 patients were enrolled (Table 1). There were no periprocedural adverse events (AEs) during EUS-FNI. All patients completed CRT without any toxicity that could be specifically attributable to R3. Two of the 6 patients with surgery had a complete pathologic response, and 4 of 6 had major pathologic response. Four patients experienced local recurrence and/or metastasis following CRT, with or without surgery. R3 was consistently visualized on verification CT scans, with retained particles observed in all patients during follow-up. No delayed AEs were noted during follow-up. Pharmacokinetic study shows R3 leakage into the bloodstream to be under the limit of detection for all patients.
Discussion: This interim analysis showed that EUS-FNI of R3 is technically feasible, the investigational drug was retained in all patients, and no periprocedural or delayed AEs were noted. Initial clinical response and outcomes are promising. Ongoing accrual and expansion will allow a more comprehensive assessment of treatment efficacy and long-term outcomes.
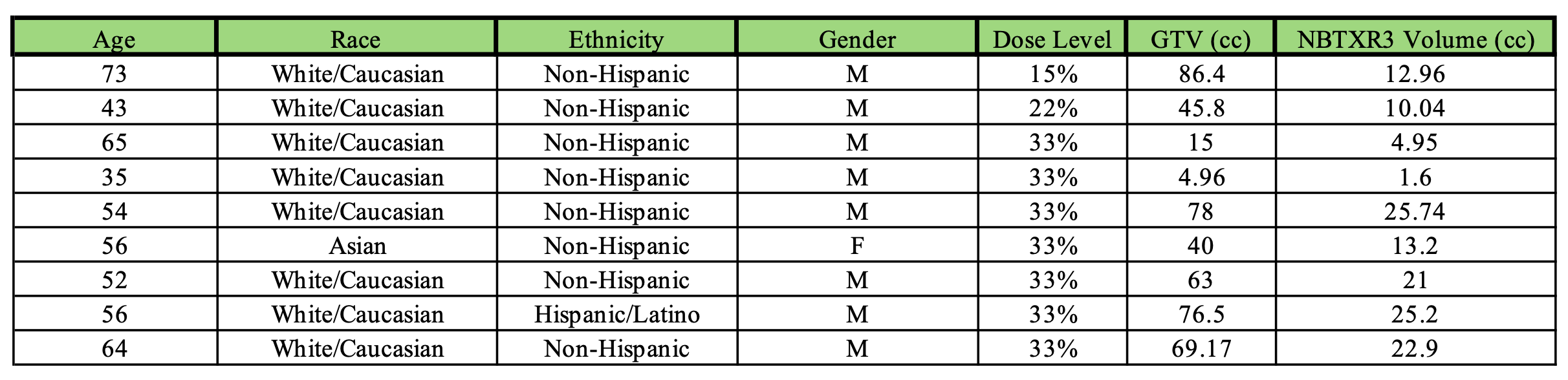
Figure: Table 1. Baseline Characteristics

Figure: Figure 1. EUS guided FNI of R3. (a) Endoscopic view of a lower esophageal adenocarcinoma. (b) EUS-FNI, multiple areas of the tumor were targeted for injection. (c) Verification CT imaging within 3 days of injection, showing retention of the investigational agent.
Disclosures:
Emmanuel Coronel indicated no relevant financial relationships.
George Wahba indicated no relevant financial relationships.
Phillip Ge: Aspero Medical – Consultant. Boston Scientific – Consultant. Fujifilm Medical Systems – Consultant. Neptune Medical – Consultant. Ovesco Endoscopy USA – Consultant. UpToDate – Royalties.
Saumil Gandhi: Johnson and Johnson – Consultant. Nanobiotix Inc – Grant/Research Support.
Juliana Bronk indicated no relevant financial relationships.
David Qian indicated no relevant financial relationships.
Joe Chang indicated no relevant financial relationships.
Michael O'Reilly indicated no relevant financial relationships.
Matthew Ning indicated no relevant financial relationships.
Aileen Chen: Pfizer/Proteus Consortium – Grant/Research Support.
Quynh-Nhu Nguyen indicated no relevant financial relationships.
Diana Amaya indicated no relevant financial relationships.
Christina Hoang indicated no relevant financial relationships.
Wayne Hofstetter indicated no relevant financial relationships.
Mariela Blum Murphy indicated no relevant financial relationships.
Albert Koong indicated no relevant financial relationships.
Zhongxing Liao indicated no relevant financial relationships.
Steven Lin: Beyond Therapeutics – Grant/Research Support. Nektar Therapeutics – Grant/Research Support. Seek Diagnostics – Co-Founder and Stock Options. ST Cube Pharmaceuticals – Grant/Research Support. XRAD Therapeutics – Consultant.
Emmanuel Coronel, MD1, George Wahba, MD2, Phillip S.. Ge, MD1, Saumil Gandhi, MD, PhD2, Juliana Bronk, MD, PhD2, David Qian, MD, PhD2, Joe Chang, MD, PhD2, Michael S. O'Reilly, MD2, Matthew Ning, MD, MPH2, Aileen B. Chen, MD2, Quynh-Nhu Nguyen, MD2, Diana Amaya, BSN2, Christina Hoang, BSN2, Wayne Hofstetter, MD2, Mariela Blum Murphy, MD2, Albert C.. Koong, MD, PhD2, Zhongxing Liao, MD, PhD2, Steven Lin, MD, PhD2. P2735 - Endoscopic Ultrasound-Guided Injection of NBTXR3 Activated by Radiotherapy With Concurrent Chemotherapy for Esophageal Adenocarcinoma: Interim Analysis of Feasibility and Safety, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1The University of Texas MD Anderson Cancer Center, Houston, TX; 2MD Anderson Cancer Center, Houston, TX
Introduction: Despite definitive chemoradiation (CRT) being the standard of care for inoperable esophageal adenocarcinoma, only 25% of patients achieve a complete treatment response. This highlights the necessity to enhance cure rates for inoperable patients and to improve clinical outcomes for operable individuals. We report the interim results of a phase I trial of endoscopic ultrasound-guided fine needle tumor injection (EUS-FNI) of NBTXR3 (R3), a hafnium oxide nanoparticle that enhances the local radiation effect by 9-fold when activated by ionizing radiation.
Methods: This is a planned interim analysis of the feasibility and safety of the first 9 patients who underwent EUS-FNI of R3 (Figure 1) and received photon therapy. Patients with bulky mediastinal tumors or tumors above the carina are excluded. Patients received escalating doses of R3, corresponding to 15%, 22%, and 33% of the gross tumor volume (GTV). Investigational drug delivery of R3 was performed under EUS guidance; the tumor was injected at multiple sites to ensure uniform distribution within the mass. A 22-gauge needle was used for EUS-FNI, and the rate of injection was 1 cc/min. Verification CT scan is taken within 3 days of the injection to visualize the distribution of the radiopaque R3 prior to starting CRT.
Results: Only one patient each was treated at 15% and 22%, with 7 treated at 33% of the GTV, a total of 9 patients were enrolled (Table 1). There were no periprocedural adverse events (AEs) during EUS-FNI. All patients completed CRT without any toxicity that could be specifically attributable to R3. Two of the 6 patients with surgery had a complete pathologic response, and 4 of 6 had major pathologic response. Four patients experienced local recurrence and/or metastasis following CRT, with or without surgery. R3 was consistently visualized on verification CT scans, with retained particles observed in all patients during follow-up. No delayed AEs were noted during follow-up. Pharmacokinetic study shows R3 leakage into the bloodstream to be under the limit of detection for all patients.
Discussion: This interim analysis showed that EUS-FNI of R3 is technically feasible, the investigational drug was retained in all patients, and no periprocedural or delayed AEs were noted. Initial clinical response and outcomes are promising. Ongoing accrual and expansion will allow a more comprehensive assessment of treatment efficacy and long-term outcomes.

Figure: Table 1. Baseline Characteristics

Figure: Figure 1. EUS guided FNI of R3. (a) Endoscopic view of a lower esophageal adenocarcinoma. (b) EUS-FNI, multiple areas of the tumor were targeted for injection. (c) Verification CT imaging within 3 days of injection, showing retention of the investigational agent.
Disclosures:
Emmanuel Coronel indicated no relevant financial relationships.
George Wahba indicated no relevant financial relationships.
Phillip Ge: Aspero Medical – Consultant. Boston Scientific – Consultant. Fujifilm Medical Systems – Consultant. Neptune Medical – Consultant. Ovesco Endoscopy USA – Consultant. UpToDate – Royalties.
Saumil Gandhi: Johnson and Johnson – Consultant. Nanobiotix Inc – Grant/Research Support.
Juliana Bronk indicated no relevant financial relationships.
David Qian indicated no relevant financial relationships.
Joe Chang indicated no relevant financial relationships.
Michael O'Reilly indicated no relevant financial relationships.
Matthew Ning indicated no relevant financial relationships.
Aileen Chen: Pfizer/Proteus Consortium – Grant/Research Support.
Quynh-Nhu Nguyen indicated no relevant financial relationships.
Diana Amaya indicated no relevant financial relationships.
Christina Hoang indicated no relevant financial relationships.
Wayne Hofstetter indicated no relevant financial relationships.
Mariela Blum Murphy indicated no relevant financial relationships.
Albert Koong indicated no relevant financial relationships.
Zhongxing Liao indicated no relevant financial relationships.
Steven Lin: Beyond Therapeutics – Grant/Research Support. Nektar Therapeutics – Grant/Research Support. Seek Diagnostics – Co-Founder and Stock Options. ST Cube Pharmaceuticals – Grant/Research Support. XRAD Therapeutics – Consultant.
Emmanuel Coronel, MD1, George Wahba, MD2, Phillip S.. Ge, MD1, Saumil Gandhi, MD, PhD2, Juliana Bronk, MD, PhD2, David Qian, MD, PhD2, Joe Chang, MD, PhD2, Michael S. O'Reilly, MD2, Matthew Ning, MD, MPH2, Aileen B. Chen, MD2, Quynh-Nhu Nguyen, MD2, Diana Amaya, BSN2, Christina Hoang, BSN2, Wayne Hofstetter, MD2, Mariela Blum Murphy, MD2, Albert C.. Koong, MD, PhD2, Zhongxing Liao, MD, PhD2, Steven Lin, MD, PhD2. P2735 - Endoscopic Ultrasound-Guided Injection of NBTXR3 Activated by Radiotherapy With Concurrent Chemotherapy for Esophageal Adenocarcinoma: Interim Analysis of Feasibility and Safety, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

