Monday Poster Session
Category: IBD
P3220 - Comparative Real-World Switch Rates of Biologics Among Patients With Crohn’s Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- JC
J. Casey Chapman, MD
GI Alliance
Dallas, Texas
Presenting Author(s)
Casey Chapman, MD1, Jenny M. Griffith, PharmD2, Bincy Abraham, MD, MS, FACG3, Nidhi Shukla, PhD4, Heather Freml, PharmD, BCPS4, Daniel O'Brien, PhD4, Gil Y. Melmed, MD, MS, FACG5
1GI Alliance, Dallas, TX; 2AbbVie Inc, North Chicago, IL; 3Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX; 4AbbVie Inc., North Chicago, IL; 5F. Widjaja Inflammatory Bowel Disease Institute, Cedars Sinai Medical Center, Los Angeles, CA
Introduction: Crohn’s disease (CD) is an immune-mediated disease characterized by relapsing and remitting gastrointestinal inflammation. Patients with CD often switch treatments due to intolerance or inadequate response. This study aims to compare switch rates of FDA approved biologics for CD over 12 months, and healthcare resource utilization (HCRU) between switchers and non-switchers.
Methods: Retrospective claims data were taken from the Merative MarketScan® database (1/2022–12/2024). Eligible patients were aged ≥18 years, with 1 inpatient or 2 outpatient medical claims for CD, but no other autoimmune conditions, and initiated a biologic approved for CD. Patients needed continuous insurance enrollment for ≥6 months prior and ≥12 months post biologic initiation. Cohorts required ≥100 patients and were mutually exclusive. Switch rate was defined as the proportion of patients switching to a new biologic within the 12-month fixed follow-up after biologic initiation. Multivariate logistic regression controlled for age, gender, insurance type, index year, prior biologic use, and baseline HCRU.
Results: Among patients receiving biologics (risankizumab [RZB], N=321; ustekinumab [UST], N=798; adalimumab [ADA], N=791; infliximab [IFX], N=637; vedolizumab [VDZ], N=424), baseline demographics were similar. At baseline, patients receiving IFX had higher rates than other cohorts of perianal abscesses (18.8% vs 3.5–9.4%) and prior IBD-related hospitalizations (17.3% vs 5.7–11.2%). Biologic-naïve patients per cohort ranged from 72.0–94.1% with RZB having the lowest and ADA having the highest. Switch rates for patients receiving RZB (9.0%) were similar to UST (10.2%) and significantly lower than ADA (20.7%), IFX (17.4%) and VDZ (16.8%) over 12 months (p< 0.05; Figure 1). Total healthcare costs were 16% higher in switchers ($132,664) than non-switchers ($114,805; Figure 2). The greatest cost difference came from medical costs, which were 65% higher in switchers ($31,714) than non-switchers ($19,277). All-cause (28.1%) and IBD-related hospitalizations (20.3%) were higher in switchers than non-switchers (all-cause, 12.9%; IBD-related, 6.3%). All-cause (35.7%) and IBD-related emergency department visits (25.7%) were also higher in switchers than non-switchers (all-cause, 24.9%; IBD-related, 12.2%).
Discussion: This real-world study shows that patients receiving RZB have the lowest switch rates, and that patients who switch treatments have higher healthcare costs and HCRU.
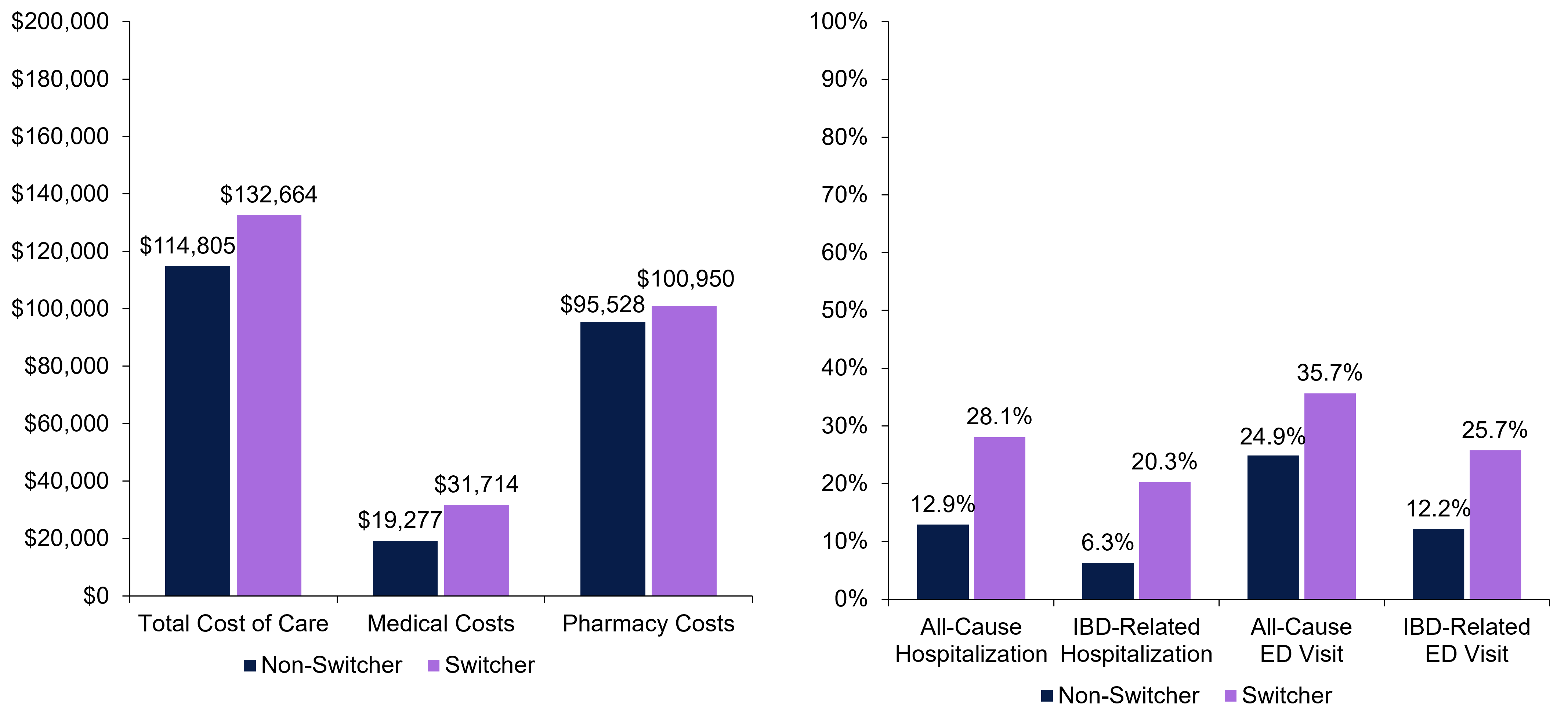
Figure: Figure 1. Switch rates by individual biologic cohort over 12 months
*P <0.05 versus risankizumab. The switch rates were calculated as (switchers/sum of switchers and non-switchers) x 100%.
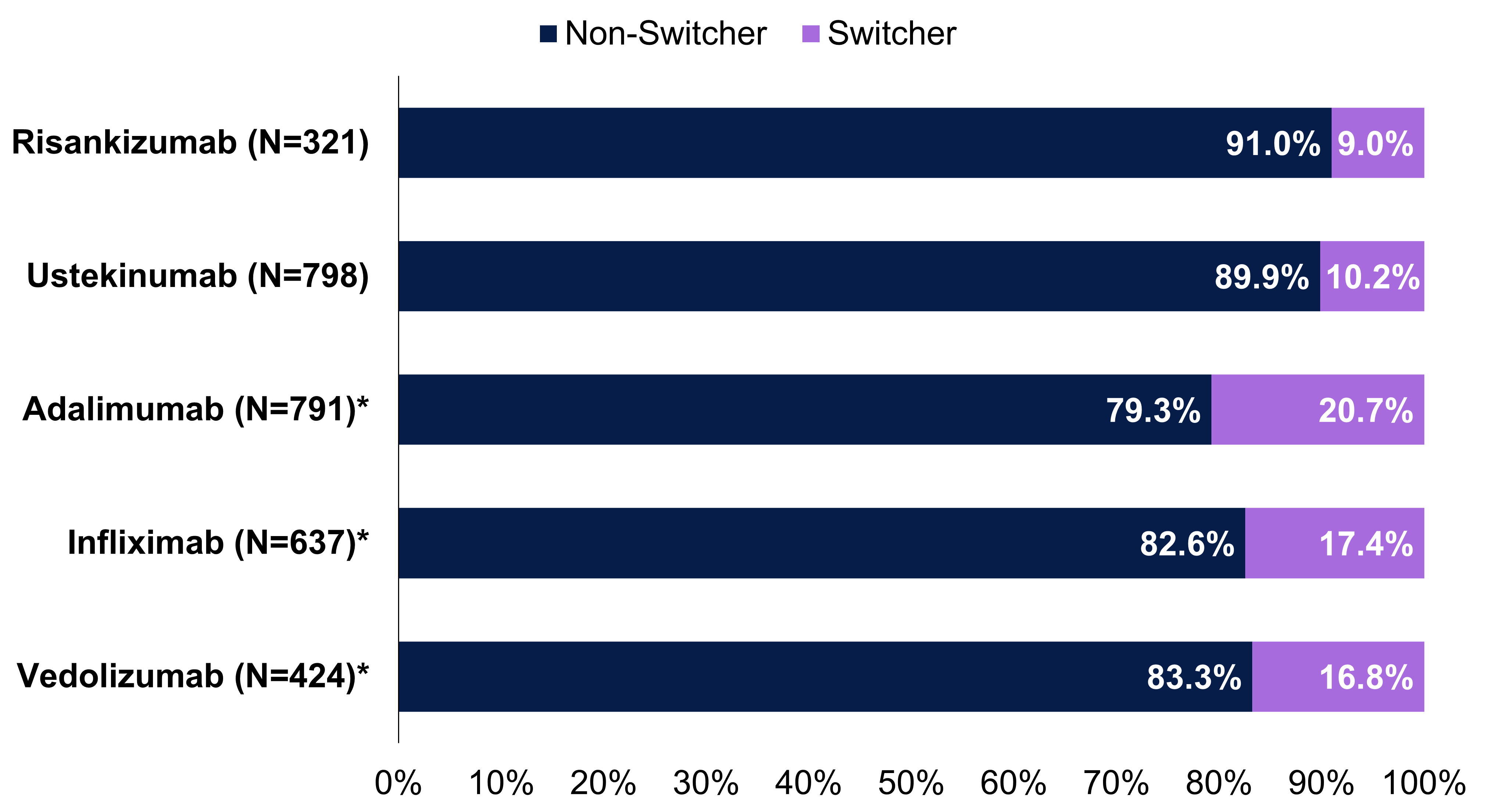
Figure: Figure 2. Healthcare costs (A) and HCRU (B) among switchers and non-switchers over 12 months
aTotal costs of care adjusted to 2025 USD. bTotal cost of care was the sum of medical costs and pharmacy costs.
ED, emergency department; HCRU, healthcare resource utilization; IBD, inflammatory bowel disease.
Disclosures:
Casey Chapman: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Speakers Bureau. Janssen – Speakers Bureau. Medtronic – Consultant. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Jenny M. Griffith: AbbVie – Employee, Stock Options.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Nidhi Shukla: AbbVie – Employee, Stock Options.
Heather Freml: AbbVie – Employee, Stock Options.
Daniel O'Brien: AbbVie Inc. – Employee, Stock Options.
Gil Y. Melmed: AbbVie – Consultant. Arena – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Dieta – Consultant, Stock-privately held company. Ferring – Consultant. Fresenius Kalbi – Consultant. Genentech – Consultant. Gilead – Consultant. Iterative Scopes – Consultant. Janssen – Consultant. Lilly – Consultant. OptionCare – Personal Fees. Oshi Health – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus Labs – Consultant. Samsung Bioepis – Consultant. Takeda – Consultant. Techlab – Consultant. Treating inflammatory bowel disease using ultraviolet light – Intellectual Property/Patents. Treating inflammatory bowel disease with antifungal therapy – Intellectual Property/Patents. Verantos – Personal Fees. Viatris – Consultant.
Casey Chapman, MD1, Jenny M. Griffith, PharmD2, Bincy Abraham, MD, MS, FACG3, Nidhi Shukla, PhD4, Heather Freml, PharmD, BCPS4, Daniel O'Brien, PhD4, Gil Y. Melmed, MD, MS, FACG5. P3220 - Comparative Real-World Switch Rates of Biologics Among Patients With Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1GI Alliance, Dallas, TX; 2AbbVie Inc, North Chicago, IL; 3Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX; 4AbbVie Inc., North Chicago, IL; 5F. Widjaja Inflammatory Bowel Disease Institute, Cedars Sinai Medical Center, Los Angeles, CA
Introduction: Crohn’s disease (CD) is an immune-mediated disease characterized by relapsing and remitting gastrointestinal inflammation. Patients with CD often switch treatments due to intolerance or inadequate response. This study aims to compare switch rates of FDA approved biologics for CD over 12 months, and healthcare resource utilization (HCRU) between switchers and non-switchers.
Methods: Retrospective claims data were taken from the Merative MarketScan® database (1/2022–12/2024). Eligible patients were aged ≥18 years, with 1 inpatient or 2 outpatient medical claims for CD, but no other autoimmune conditions, and initiated a biologic approved for CD. Patients needed continuous insurance enrollment for ≥6 months prior and ≥12 months post biologic initiation. Cohorts required ≥100 patients and were mutually exclusive. Switch rate was defined as the proportion of patients switching to a new biologic within the 12-month fixed follow-up after biologic initiation. Multivariate logistic regression controlled for age, gender, insurance type, index year, prior biologic use, and baseline HCRU.
Results: Among patients receiving biologics (risankizumab [RZB], N=321; ustekinumab [UST], N=798; adalimumab [ADA], N=791; infliximab [IFX], N=637; vedolizumab [VDZ], N=424), baseline demographics were similar. At baseline, patients receiving IFX had higher rates than other cohorts of perianal abscesses (18.8% vs 3.5–9.4%) and prior IBD-related hospitalizations (17.3% vs 5.7–11.2%). Biologic-naïve patients per cohort ranged from 72.0–94.1% with RZB having the lowest and ADA having the highest. Switch rates for patients receiving RZB (9.0%) were similar to UST (10.2%) and significantly lower than ADA (20.7%), IFX (17.4%) and VDZ (16.8%) over 12 months (p< 0.05; Figure 1). Total healthcare costs were 16% higher in switchers ($132,664) than non-switchers ($114,805; Figure 2). The greatest cost difference came from medical costs, which were 65% higher in switchers ($31,714) than non-switchers ($19,277). All-cause (28.1%) and IBD-related hospitalizations (20.3%) were higher in switchers than non-switchers (all-cause, 12.9%; IBD-related, 6.3%). All-cause (35.7%) and IBD-related emergency department visits (25.7%) were also higher in switchers than non-switchers (all-cause, 24.9%; IBD-related, 12.2%).
Discussion: This real-world study shows that patients receiving RZB have the lowest switch rates, and that patients who switch treatments have higher healthcare costs and HCRU.
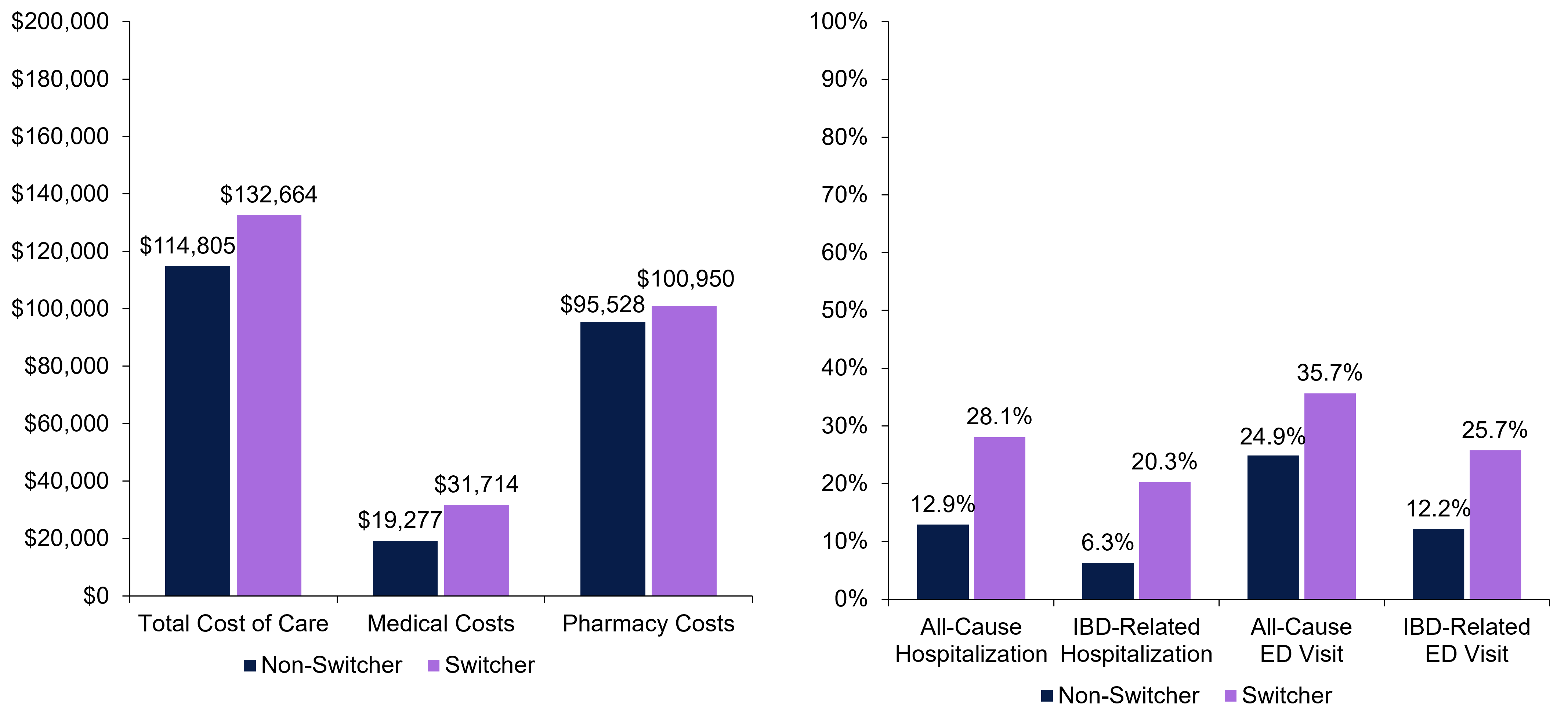
Figure: Figure 1. Switch rates by individual biologic cohort over 12 months
*P <0.05 versus risankizumab. The switch rates were calculated as (switchers/sum of switchers and non-switchers) x 100%.

Figure: Figure 2. Healthcare costs (A) and HCRU (B) among switchers and non-switchers over 12 months
aTotal costs of care adjusted to 2025 USD. bTotal cost of care was the sum of medical costs and pharmacy costs.
ED, emergency department; HCRU, healthcare resource utilization; IBD, inflammatory bowel disease.
Disclosures:
Casey Chapman: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Speakers Bureau. Janssen – Speakers Bureau. Medtronic – Consultant. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Jenny M. Griffith: AbbVie – Employee, Stock Options.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Nidhi Shukla: AbbVie – Employee, Stock Options.
Heather Freml: AbbVie – Employee, Stock Options.
Daniel O'Brien: AbbVie Inc. – Employee, Stock Options.
Gil Y. Melmed: AbbVie – Consultant. Arena – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Dieta – Consultant, Stock-privately held company. Ferring – Consultant. Fresenius Kalbi – Consultant. Genentech – Consultant. Gilead – Consultant. Iterative Scopes – Consultant. Janssen – Consultant. Lilly – Consultant. OptionCare – Personal Fees. Oshi Health – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus Labs – Consultant. Samsung Bioepis – Consultant. Takeda – Consultant. Techlab – Consultant. Treating inflammatory bowel disease using ultraviolet light – Intellectual Property/Patents. Treating inflammatory bowel disease with antifungal therapy – Intellectual Property/Patents. Verantos – Personal Fees. Viatris – Consultant.
Casey Chapman, MD1, Jenny M. Griffith, PharmD2, Bincy Abraham, MD, MS, FACG3, Nidhi Shukla, PhD4, Heather Freml, PharmD, BCPS4, Daniel O'Brien, PhD4, Gil Y. Melmed, MD, MS, FACG5. P3220 - Comparative Real-World Switch Rates of Biologics Among Patients With Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
