Monday Poster Session
Category: IBD
P3279 - Effectiveness of Upadacitinib in Ulcerative Colitis Patients With Prior Tofacitinib Exposure: A Systematic Review and Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- KK
Kinza Khan, MD (she/her/hers)
University of Pennsylvania Health System
Philadelphia, PA
Presenting Author(s)
Fariha Hasan, MD1, Ayesha Liaquat, MBBS2, Kinza Khan, MD3, Fatima Farooqi, MBBS4, Zain Ali Nadeem, 5, Daniel Guirguis, MD, MS6, Aizaz Ali, MBBS7, Hassam Ali, MD8, Hareesha Rishab Bharadwaj, 9, Shahryar Khan, MD10, Rachel Frank, MD11, Gursimran Kochhar, MD12
1Cooper University Hospital, Camden, NJ; 2Dow Medical College, Karachi, Sindh, Pakistan; 3University of Pennsylvania Health System, Philadelphia, PA; 4Dow University of Health Sciences, Karachi, Sindh, Pakistan; 5Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 6St. Luke's University Hospital, Hatfield, PA; 7Khyber Medical college, Peshawar, Mardan, North-West Frontier, Pakistan; 8East Carolina University/Brody School of Medicine, Greenville, NC; 9The University of Manchester, Manchester, England, United Kingdom; 10University of Kansas Medical Center, Kansas City, KS; 11Cooper University Hospital, Philadelphia, PA; 12Allegheny Health Network, Pittsburgh, PA
Introduction: Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by diarrhea, abdominal pain, hematochezia, and tenesmus. Over the past two decades, several biologics and small-molecule therapies have been approved for the treatment of moderate-to-severe UC, including Upadacitinib, an oral selective small-molecule Janus Kinase (JAK) inhibitor, which was approved by the US Food and Drug Administration (FDA) in March 2022. However, limited data is available on its efficacy in patients previously exposed to Tofacitinib, a first-generation non-selective oral JAK inhibitor. Therefore, we conducted a systematic review and meta-analysis to evaluate the efficacy of Upadacitinib in UC patients with prior tofacitinib treatment.
Methods: Following Cochrane and PRISMA guidelines, we systematically reviewed four databases; PubMed, Embase, Web of Science, and the Cochrane Library, for studies evaluating the effectiveness of Upadacitinib in UC patients with prior exposure to Tofacitinib. Statistical analyses were performed using R version 4.4.1, calculating pooled proportions with 95% confidence intervals (CIs) for dichotomous outcomes and mean differences (MDs) with 95% CIs for continuous outcomes using a random-effects model. Proportional data were transformed using the Freeman-Tukey double-arcsine method, and variance was calculated using the restricted maximum-likelihood estimator.
Results: We included 6 studies in our final analysis. All included studies evaluated the effectiveness of Upadacitinib in UC patients previously treated with tofacitinib. The meta-analysis revealed that Upadacitinib increased pooled clinical remission rates by 57% (95% CI: 0.32–0.80; I2 = 84%; P < 0.01), steroid-free clinical remission rates by 52% (95% CI: 0.33–0.72; I2 = 61%; P = 0.05), and clinical response rates by 70% (95% CI: 0.57–0.82; I2 = 35%; P = 0.21). Additionally, Upadacitinib reduced mean fecal calprotectin levels by 593.19 points (95% CI: 349.06–837.31; I2 = 35%; P = 0.21). Adverse events associated with Upadacitinib, such as headache, acne vulgaris, rash, nasopharyngitis, and infections, were reported in 31% of patients (95% CI: 0.14–0.51; I2 = 71%; P = 0.02).
Discussion: Our meta-analysis indicates that Upadacitinib may be an effective treatment for patients with prior tofacitinib exposure, as it achieves significant clinical remission, steroid-free clinical remission, and clinical response. Larger prospective studies are needed to confirm and validate these findings.
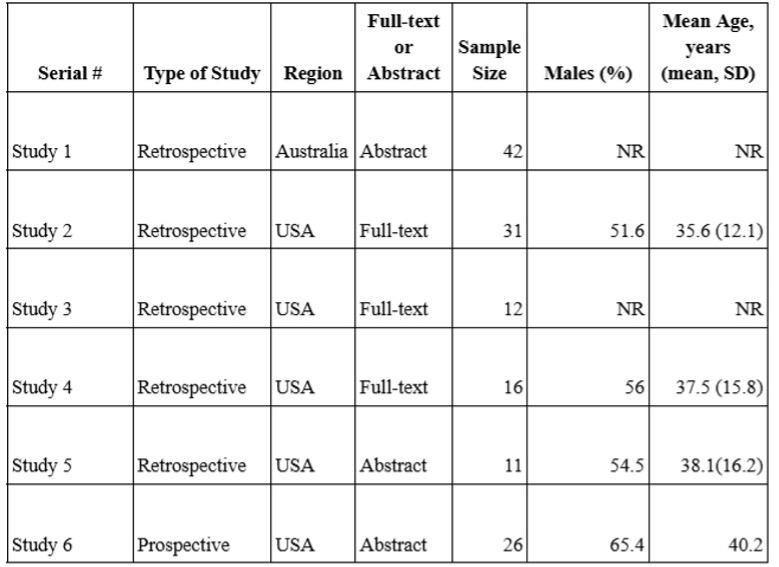
Figure: Table 1. Summary of Included Studies
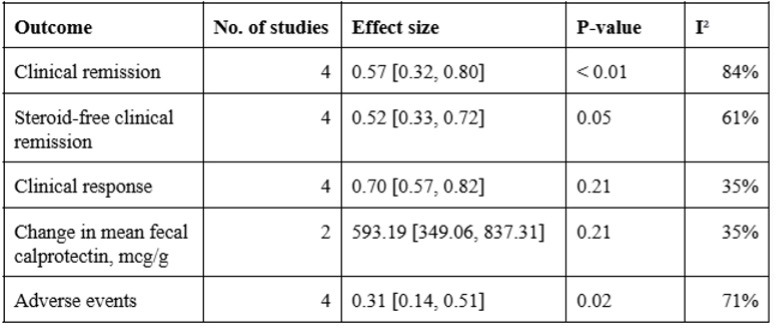
Figure: Table 2. Pooled Outcomes From Included Studies
Disclosures:
Fariha Hasan indicated no relevant financial relationships.
Ayesha Liaquat indicated no relevant financial relationships.
Kinza Khan indicated no relevant financial relationships.
Fatima Farooqi indicated no relevant financial relationships.
Zain Ali Nadeem indicated no relevant financial relationships.
Daniel Guirguis indicated no relevant financial relationships.
Aizaz Ali indicated no relevant financial relationships.
Hassam Ali indicated no relevant financial relationships.
Hareesha Rishab Bharadwaj indicated no relevant financial relationships.
Shahryar Khan indicated no relevant financial relationships.
Rachel Frank indicated no relevant financial relationships.
Gursimran Kochhar indicated no relevant financial relationships.
Fariha Hasan, MD1, Ayesha Liaquat, MBBS2, Kinza Khan, MD3, Fatima Farooqi, MBBS4, Zain Ali Nadeem, 5, Daniel Guirguis, MD, MS6, Aizaz Ali, MBBS7, Hassam Ali, MD8, Hareesha Rishab Bharadwaj, 9, Shahryar Khan, MD10, Rachel Frank, MD11, Gursimran Kochhar, MD12. P3279 - Effectiveness of Upadacitinib in Ulcerative Colitis Patients With Prior Tofacitinib Exposure: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cooper University Hospital, Camden, NJ; 2Dow Medical College, Karachi, Sindh, Pakistan; 3University of Pennsylvania Health System, Philadelphia, PA; 4Dow University of Health Sciences, Karachi, Sindh, Pakistan; 5Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 6St. Luke's University Hospital, Hatfield, PA; 7Khyber Medical college, Peshawar, Mardan, North-West Frontier, Pakistan; 8East Carolina University/Brody School of Medicine, Greenville, NC; 9The University of Manchester, Manchester, England, United Kingdom; 10University of Kansas Medical Center, Kansas City, KS; 11Cooper University Hospital, Philadelphia, PA; 12Allegheny Health Network, Pittsburgh, PA
Introduction: Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by diarrhea, abdominal pain, hematochezia, and tenesmus. Over the past two decades, several biologics and small-molecule therapies have been approved for the treatment of moderate-to-severe UC, including Upadacitinib, an oral selective small-molecule Janus Kinase (JAK) inhibitor, which was approved by the US Food and Drug Administration (FDA) in March 2022. However, limited data is available on its efficacy in patients previously exposed to Tofacitinib, a first-generation non-selective oral JAK inhibitor. Therefore, we conducted a systematic review and meta-analysis to evaluate the efficacy of Upadacitinib in UC patients with prior tofacitinib treatment.
Methods: Following Cochrane and PRISMA guidelines, we systematically reviewed four databases; PubMed, Embase, Web of Science, and the Cochrane Library, for studies evaluating the effectiveness of Upadacitinib in UC patients with prior exposure to Tofacitinib. Statistical analyses were performed using R version 4.4.1, calculating pooled proportions with 95% confidence intervals (CIs) for dichotomous outcomes and mean differences (MDs) with 95% CIs for continuous outcomes using a random-effects model. Proportional data were transformed using the Freeman-Tukey double-arcsine method, and variance was calculated using the restricted maximum-likelihood estimator.
Results: We included 6 studies in our final analysis. All included studies evaluated the effectiveness of Upadacitinib in UC patients previously treated with tofacitinib. The meta-analysis revealed that Upadacitinib increased pooled clinical remission rates by 57% (95% CI: 0.32–0.80; I2 = 84%; P < 0.01), steroid-free clinical remission rates by 52% (95% CI: 0.33–0.72; I2 = 61%; P = 0.05), and clinical response rates by 70% (95% CI: 0.57–0.82; I2 = 35%; P = 0.21). Additionally, Upadacitinib reduced mean fecal calprotectin levels by 593.19 points (95% CI: 349.06–837.31; I2 = 35%; P = 0.21). Adverse events associated with Upadacitinib, such as headache, acne vulgaris, rash, nasopharyngitis, and infections, were reported in 31% of patients (95% CI: 0.14–0.51; I2 = 71%; P = 0.02).
Discussion: Our meta-analysis indicates that Upadacitinib may be an effective treatment for patients with prior tofacitinib exposure, as it achieves significant clinical remission, steroid-free clinical remission, and clinical response. Larger prospective studies are needed to confirm and validate these findings.

Figure: Table 1. Summary of Included Studies

Figure: Table 2. Pooled Outcomes From Included Studies
Disclosures:
Fariha Hasan indicated no relevant financial relationships.
Ayesha Liaquat indicated no relevant financial relationships.
Kinza Khan indicated no relevant financial relationships.
Fatima Farooqi indicated no relevant financial relationships.
Zain Ali Nadeem indicated no relevant financial relationships.
Daniel Guirguis indicated no relevant financial relationships.
Aizaz Ali indicated no relevant financial relationships.
Hassam Ali indicated no relevant financial relationships.
Hareesha Rishab Bharadwaj indicated no relevant financial relationships.
Shahryar Khan indicated no relevant financial relationships.
Rachel Frank indicated no relevant financial relationships.
Gursimran Kochhar indicated no relevant financial relationships.
Fariha Hasan, MD1, Ayesha Liaquat, MBBS2, Kinza Khan, MD3, Fatima Farooqi, MBBS4, Zain Ali Nadeem, 5, Daniel Guirguis, MD, MS6, Aizaz Ali, MBBS7, Hassam Ali, MD8, Hareesha Rishab Bharadwaj, 9, Shahryar Khan, MD10, Rachel Frank, MD11, Gursimran Kochhar, MD12. P3279 - Effectiveness of Upadacitinib in Ulcerative Colitis Patients With Prior Tofacitinib Exposure: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
