Monday Poster Session
Category: Infections and Microbiome
P3442 - CFTR Modulators Are Not Associated With Reduction in Cancer Incidence: A Propensity Matched Analysis of 15,280 Cystic Fibrosis Patients
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- PY
Pradeep Yarra, MD (he/him/his)
Saint Louis University School of Medicine
Saint Louis, MO
Presenting Author(s)
Award: ACG Outstanding Research Award in the Infections and Microbiome Category (Trainee)
Award: ACG Presidential Poster Award
Pradeep Yarra, MD1, Yassine Kilani, MD1, Shravya R. Ginnaram, MD2, Rohan Tripathi, MD1, Alfred Nelson, MD1, Christine Hachem, MD, FACG1
1Saint Louis University School of Medicine, Saint Louis, MO; 2University of Nebraska Medical Center, Omaha, NE
Introduction: Cystic fibrosis trans membrane conductance regulator (CFTR) modulators have been shown to significantly improve the clinical outcomes and life expectancy of patients with cystic fibrosis (CF). However, the long term safety of these medications, particularly regarding to cancer risk or benefit, remains an area of ongoing investigation. We aimed to assess the long-term cancer risk and/or benefit with CFTR modulators.
Methods: We conducted a retrospective cohort study in the United States using the TriNetX Research Network to identify patients with CF, then stratified into two groups: CF patients treated with CFTR modulators (Deutivacaftor, Elexacaftor, Ivafactor, Lumacaftor, Tezacaftor and Vanzacaftor) within 3 years after CF diagnosis and those who were not (controls). Cohorts were matched using one-to-one propensity score matching on demographics, comorbidities, family history of malignancy, social determinants, treatments, and lung transplant. Kaplan Meier analysis, and Cox regression analysis were used to assess the 10-year outcomes, including new-onset cancer, gastrointestinal or hepatobiliary cancers, all-cause mortality, and all-cause hospitalizations among CF patients using CFTR modulators compared to controls.
Results: A total of 7640 patients with CF treated with CFTR modulators were propensity matched with 7640 controls. Among unmatched samples, patients in the treatment group were younger, more males, White, with higher rates of malnutrition, and lower rates of smoking and family history of malignancy (all p < 0.0001). Furthermore, CF patients who recieved CFTR modulators were more likely to be treated with antibiotics and less likely to be on IS agents (p < 0.0001). Among matched samples, there were no significant differences in the overall risk of cancer (3.7% vs. 3.9%, HR 1.028, 95%CI: 0.859-1.231) and risk of gastrointestinal cancers (0.3% vs. 0.2%, HR 1.684, 95%CI: 0.835-3.393); however, patients on CFTR modulators were at lower risk of 10-year mortality (1.0% vs. 3.1%, HR 0.357, 95%CI: 0.277-0.461), and higher risk of all-cause hospitalization (13.0% vs. 9.5%, HR 1.364, 95%CI 1.239-1.503).
Discussion: Our study showed that there is no conclusive evidence linking CFTR modulators to cancer prevention in CF patients, although their use correlated with reduced mortality, and increased healthcare resource utilization. Additional long-term prospective studies are required to further understand the association between CFTR modulators and cancer risk.
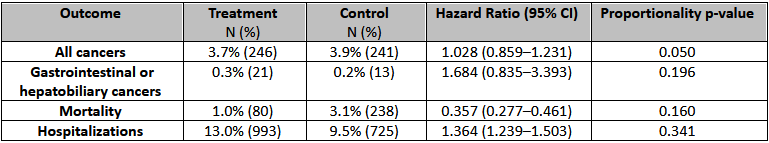
Figure: Baseline characteristics; SDHO: Social determinants of adverse health outcomes
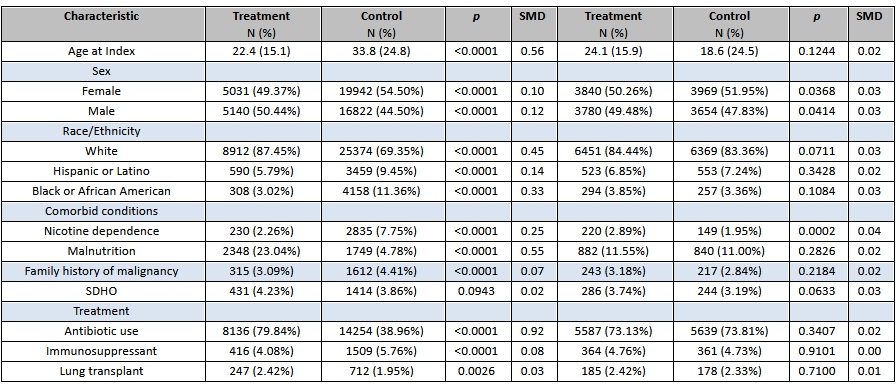
Figure: Table 2. Outcomes of CF patients treated with CFTR modulators compared to controls
Disclosures:
Pradeep Yarra indicated no relevant financial relationships.
Yassine Kilani indicated no relevant financial relationships.
Shravya Ginnaram indicated no relevant financial relationships.
Rohan Tripathi indicated no relevant financial relationships.
Alfred Nelson indicated no relevant financial relationships.
Christine Hachem indicated no relevant financial relationships.
Pradeep Yarra, MD1, Yassine Kilani, MD1, Shravya R. Ginnaram, MD2, Rohan Tripathi, MD1, Alfred Nelson, MD1, Christine Hachem, MD, FACG1. P3442 - CFTR Modulators Are Not Associated With Reduction in Cancer Incidence: A Propensity Matched Analysis of 15,280 Cystic Fibrosis Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Award: ACG Presidential Poster Award
Pradeep Yarra, MD1, Yassine Kilani, MD1, Shravya R. Ginnaram, MD2, Rohan Tripathi, MD1, Alfred Nelson, MD1, Christine Hachem, MD, FACG1
1Saint Louis University School of Medicine, Saint Louis, MO; 2University of Nebraska Medical Center, Omaha, NE
Introduction: Cystic fibrosis trans membrane conductance regulator (CFTR) modulators have been shown to significantly improve the clinical outcomes and life expectancy of patients with cystic fibrosis (CF). However, the long term safety of these medications, particularly regarding to cancer risk or benefit, remains an area of ongoing investigation. We aimed to assess the long-term cancer risk and/or benefit with CFTR modulators.
Methods: We conducted a retrospective cohort study in the United States using the TriNetX Research Network to identify patients with CF, then stratified into two groups: CF patients treated with CFTR modulators (Deutivacaftor, Elexacaftor, Ivafactor, Lumacaftor, Tezacaftor and Vanzacaftor) within 3 years after CF diagnosis and those who were not (controls). Cohorts were matched using one-to-one propensity score matching on demographics, comorbidities, family history of malignancy, social determinants, treatments, and lung transplant. Kaplan Meier analysis, and Cox regression analysis were used to assess the 10-year outcomes, including new-onset cancer, gastrointestinal or hepatobiliary cancers, all-cause mortality, and all-cause hospitalizations among CF patients using CFTR modulators compared to controls.
Results: A total of 7640 patients with CF treated with CFTR modulators were propensity matched with 7640 controls. Among unmatched samples, patients in the treatment group were younger, more males, White, with higher rates of malnutrition, and lower rates of smoking and family history of malignancy (all p < 0.0001). Furthermore, CF patients who recieved CFTR modulators were more likely to be treated with antibiotics and less likely to be on IS agents (p < 0.0001). Among matched samples, there were no significant differences in the overall risk of cancer (3.7% vs. 3.9%, HR 1.028, 95%CI: 0.859-1.231) and risk of gastrointestinal cancers (0.3% vs. 0.2%, HR 1.684, 95%CI: 0.835-3.393); however, patients on CFTR modulators were at lower risk of 10-year mortality (1.0% vs. 3.1%, HR 0.357, 95%CI: 0.277-0.461), and higher risk of all-cause hospitalization (13.0% vs. 9.5%, HR 1.364, 95%CI 1.239-1.503).
Discussion: Our study showed that there is no conclusive evidence linking CFTR modulators to cancer prevention in CF patients, although their use correlated with reduced mortality, and increased healthcare resource utilization. Additional long-term prospective studies are required to further understand the association between CFTR modulators and cancer risk.

Figure: Baseline characteristics; SDHO: Social determinants of adverse health outcomes

Figure: Table 2. Outcomes of CF patients treated with CFTR modulators compared to controls
Disclosures:
Pradeep Yarra indicated no relevant financial relationships.
Yassine Kilani indicated no relevant financial relationships.
Shravya Ginnaram indicated no relevant financial relationships.
Rohan Tripathi indicated no relevant financial relationships.
Alfred Nelson indicated no relevant financial relationships.
Christine Hachem indicated no relevant financial relationships.
Pradeep Yarra, MD1, Yassine Kilani, MD1, Shravya R. Ginnaram, MD2, Rohan Tripathi, MD1, Alfred Nelson, MD1, Christine Hachem, MD, FACG1. P3442 - CFTR Modulators Are Not Associated With Reduction in Cancer Incidence: A Propensity Matched Analysis of 15,280 Cystic Fibrosis Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

