Monday Poster Session
Category: Liver
P3688 - Redefining Cirrhosis Care: The Role of SGLT2 Inhibitors in Managing Ascites, SBP, and TIPS Interventions
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Chidera Onwuzo, MBBS (he/him/his)
SUNY Upstate Medical University Hospital
Syracuse, NY
Presenting Author(s)
Chidera Onwuzo, MBBS1, Somtochukwu Onwuzo, MD2, Kojo-Frimpong B. Awuah, MD3, Mathew Chiong, MD1, Rashid Abdel-Razeq, MD4, Laith Alomari, MD5, Al-Aman Shaukat, MBBS1
1SUNY Upstate Medical University Hospital, Syracuse, NY; 2Allegheny Center for Digestive Health, Pittsburgh, PA; 3Allegheny Health Network, Pittsburgh, PA; 4Cleveland Clinic Foundation, Cleveland, OH; 5Thomas Jefferson University, Philadelphia, PA
Introduction: Ascites, spontaneous bacterial peritonitis (SBP), and the need for therapeutic treatments like paracentesis and transjugular intrahepatic portosystemic shunt (TIPS) are just a few of the consequences that can arise from cirrhosis. Although the main use of SGLT-2 inhibitors is in the treatment of type 2 diabetes and decreasing mortality in advanced heart failure, new research indicates that these drugs may also be very beneficial in the treatment of cirrhosis by reducing certain consequences. The objective of this study is to thoroughly assess the impact of SGLT-2 medication on these particular clinical outcomes in cirrhosis patients in order to further our comprehension of the therapeutic implications of this intricate patient population.
Methods: We conducted a retrospective analysis using deidentified, aggregate patient data from the TriNetX research network. The cohort comprised patients aged ≥18 years with a diagnosis of cirrhosis. Patients who commenced on SGLT-2 inhibitors after the diagnosis were identified. Primary outcomes included ascites, the need for paracentesis, SBP, and TIPS procedures. Variables such as age, sex, race, comorbidities (e.g., diabetes, obesity, chronic kidney disease), and lifestyle factors (e.g., tobacco use, alcohol dependence) underwent 1:1 propensity matching to mitigate confounding. Odds ratios (OR) with 95% confidence intervals (CI) were reported.
Results: The matched cohort analysis revealed that SGLT-2 inhibitor use was significantly associated with a reduced risk of key cirrhosis-related complications. There was a significant reduction in the incidence of ascites (OR 0.545, 95% CI 0.517–0.575), the need for paracentesis (OR 0.458, 95% CI 0.426–0.493), and SBP (OR 0.443, 95% CI 0.384–0.510). Additionally, the likelihood of requiring TIPS was significantly lower in patients receiving SGLT-2 inhibitors (OR 0.243, 95% CI 0.122–0.486).
Discussion: This study shows that in patients with cirrhosis, SGLT-2 inhibitors are strongly linked to a lower frequency of ascites, the need for paracentesis, spontaneous bacterial peritonitis (SBP), and the necessity for transjugular intrahepatic portosystemic shunts (TIPS). These strong results highlight the potential of SGLT-2 therapy to improve clinical outcomes and efficiently treat cirrhosis-related comorbidities. This suggests that SGLT-2 inhibitors may play a transformative role in the therapeutic landscape for cirrhotic patients, paving the way for improved patient care and management strategies.
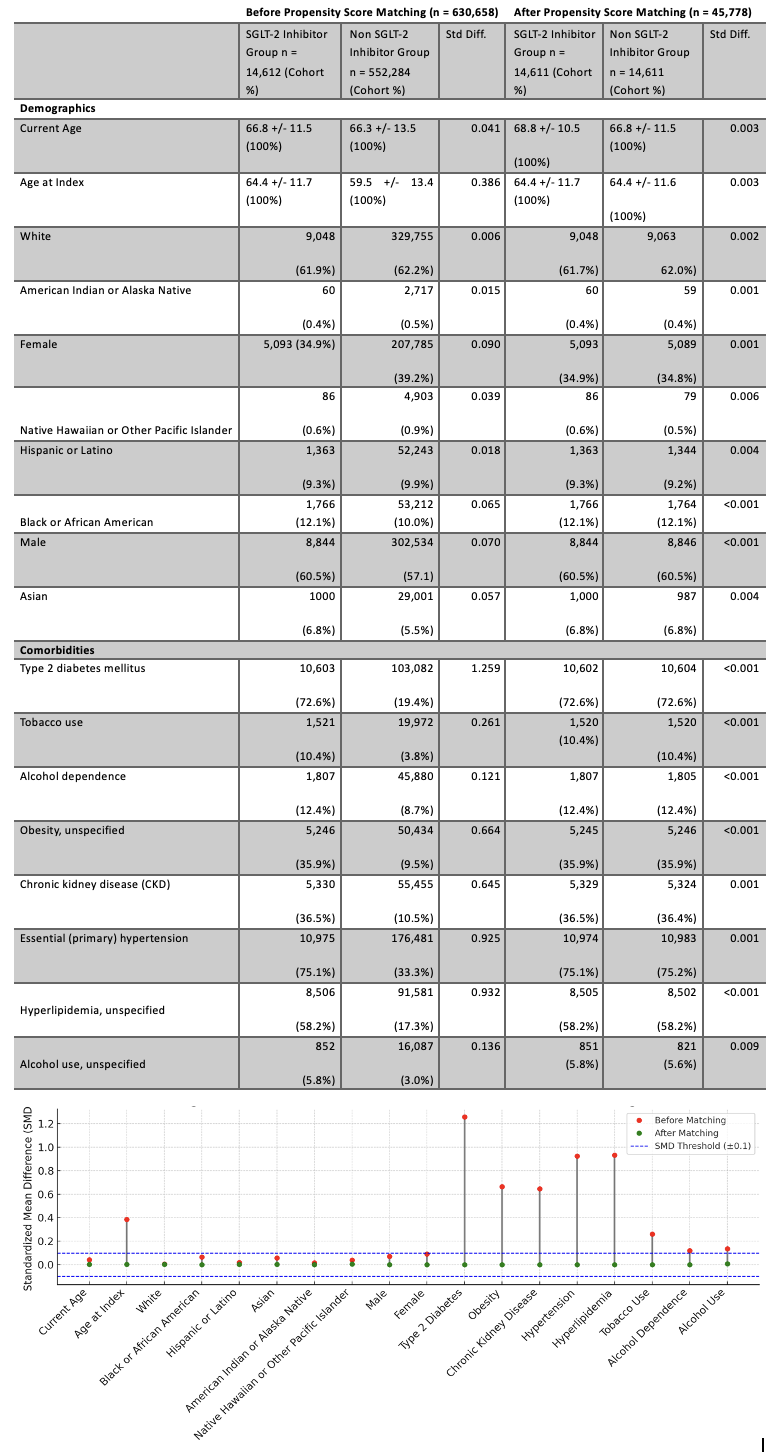
Figure: Figure 1: Comparison of Demographics and Comorbidities of SGLT-2 Inhibitors Group vs Non SGLT-2 Inhibitors Group Before and After Propensity Score Matching
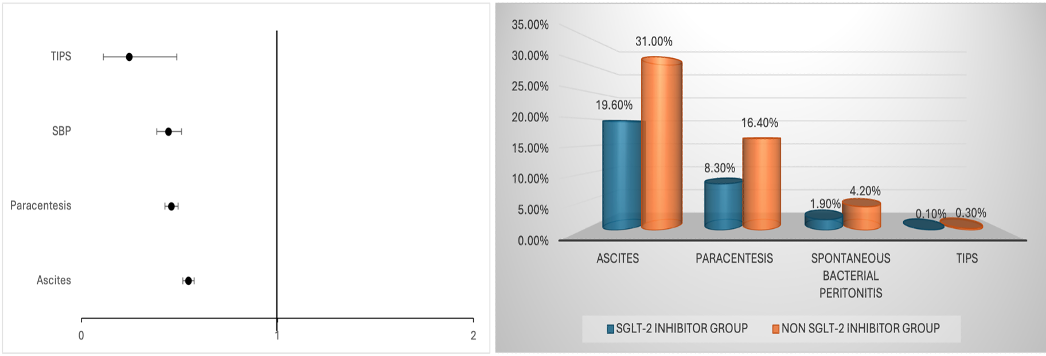
Figure: Figure 2:: Forest plot comparing odds ratios for events & risk of complications in SGLT-2 Inhibitors Group vs. Non SGLT-2 Inhibitors Group in patients with cirrhosis.
Disclosures:
Chidera Onwuzo indicated no relevant financial relationships.
Somtochukwu Onwuzo indicated no relevant financial relationships.
Kojo-Frimpong B. Awuah indicated no relevant financial relationships.
Mathew Chiong indicated no relevant financial relationships.
Rashid Abdel-Razeq indicated no relevant financial relationships.
Laith Alomari indicated no relevant financial relationships.
Al-Aman Shaukat indicated no relevant financial relationships.
Chidera Onwuzo, MBBS1, Somtochukwu Onwuzo, MD2, Kojo-Frimpong B. Awuah, MD3, Mathew Chiong, MD1, Rashid Abdel-Razeq, MD4, Laith Alomari, MD5, Al-Aman Shaukat, MBBS1. P3688 - Redefining Cirrhosis Care: The Role of SGLT2 Inhibitors in Managing Ascites, SBP, and TIPS Interventions, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1SUNY Upstate Medical University Hospital, Syracuse, NY; 2Allegheny Center for Digestive Health, Pittsburgh, PA; 3Allegheny Health Network, Pittsburgh, PA; 4Cleveland Clinic Foundation, Cleveland, OH; 5Thomas Jefferson University, Philadelphia, PA
Introduction: Ascites, spontaneous bacterial peritonitis (SBP), and the need for therapeutic treatments like paracentesis and transjugular intrahepatic portosystemic shunt (TIPS) are just a few of the consequences that can arise from cirrhosis. Although the main use of SGLT-2 inhibitors is in the treatment of type 2 diabetes and decreasing mortality in advanced heart failure, new research indicates that these drugs may also be very beneficial in the treatment of cirrhosis by reducing certain consequences. The objective of this study is to thoroughly assess the impact of SGLT-2 medication on these particular clinical outcomes in cirrhosis patients in order to further our comprehension of the therapeutic implications of this intricate patient population.
Methods: We conducted a retrospective analysis using deidentified, aggregate patient data from the TriNetX research network. The cohort comprised patients aged ≥18 years with a diagnosis of cirrhosis. Patients who commenced on SGLT-2 inhibitors after the diagnosis were identified. Primary outcomes included ascites, the need for paracentesis, SBP, and TIPS procedures. Variables such as age, sex, race, comorbidities (e.g., diabetes, obesity, chronic kidney disease), and lifestyle factors (e.g., tobacco use, alcohol dependence) underwent 1:1 propensity matching to mitigate confounding. Odds ratios (OR) with 95% confidence intervals (CI) were reported.
Results: The matched cohort analysis revealed that SGLT-2 inhibitor use was significantly associated with a reduced risk of key cirrhosis-related complications. There was a significant reduction in the incidence of ascites (OR 0.545, 95% CI 0.517–0.575), the need for paracentesis (OR 0.458, 95% CI 0.426–0.493), and SBP (OR 0.443, 95% CI 0.384–0.510). Additionally, the likelihood of requiring TIPS was significantly lower in patients receiving SGLT-2 inhibitors (OR 0.243, 95% CI 0.122–0.486).
Discussion: This study shows that in patients with cirrhosis, SGLT-2 inhibitors are strongly linked to a lower frequency of ascites, the need for paracentesis, spontaneous bacterial peritonitis (SBP), and the necessity for transjugular intrahepatic portosystemic shunts (TIPS). These strong results highlight the potential of SGLT-2 therapy to improve clinical outcomes and efficiently treat cirrhosis-related comorbidities. This suggests that SGLT-2 inhibitors may play a transformative role in the therapeutic landscape for cirrhotic patients, paving the way for improved patient care and management strategies.

Figure: Figure 1: Comparison of Demographics and Comorbidities of SGLT-2 Inhibitors Group vs Non SGLT-2 Inhibitors Group Before and After Propensity Score Matching

Figure: Figure 2:: Forest plot comparing odds ratios for events & risk of complications in SGLT-2 Inhibitors Group vs. Non SGLT-2 Inhibitors Group in patients with cirrhosis.
Disclosures:
Chidera Onwuzo indicated no relevant financial relationships.
Somtochukwu Onwuzo indicated no relevant financial relationships.
Kojo-Frimpong B. Awuah indicated no relevant financial relationships.
Mathew Chiong indicated no relevant financial relationships.
Rashid Abdel-Razeq indicated no relevant financial relationships.
Laith Alomari indicated no relevant financial relationships.
Al-Aman Shaukat indicated no relevant financial relationships.
Chidera Onwuzo, MBBS1, Somtochukwu Onwuzo, MD2, Kojo-Frimpong B. Awuah, MD3, Mathew Chiong, MD1, Rashid Abdel-Razeq, MD4, Laith Alomari, MD5, Al-Aman Shaukat, MBBS1. P3688 - Redefining Cirrhosis Care: The Role of SGLT2 Inhibitors in Managing Ascites, SBP, and TIPS Interventions, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
