Monday Poster Session
Category: Liver
P3677 - Retrospective Analysis of Hepatitis B Reactivation in Patients Undergoing Pembrolizumab Treatment by Country
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- BL
Brian Li, DO
University of Florida College of Medicine
Jacksonville, FL
Presenting Author(s)
Brian Li, DO, Abha Sathe, MD, Kabeer Ali, MD, Charles J. Harrison, MD
University of Florida College of Medicine, Jacksonville, FL
Introduction: Our objective is to analyze the risk of hepatitis B reactivation in patients undergoing Pembrolizumab therapy based on country.
Methods: Data from the FDA Adverse Events Reporting System (FAERS) public dashboard was collected on hepatitis B reactivation in patients undergoing Pembrolizumab monotherapy. Data was also collected from FAERS to assess the total number of reported hepatitis B reactivations since 2014, when pembrolizumab was introduced. Both data sets were used to determine a relative risk for hepatitis B reactivation based on the reporting country.
Results: From 2014 to 2024, 3472 cases of hepatitis B reactivation were reported to the FAERS dashboard. 13 cases were reported in patients undergoing pembrolizumab treatment, approximately 0.37% of all cases. Of the 13 total cases, 7 (53.8%) were from the United States (US), 2 (15.4%) were from China (CN), and 1 each (7.7%) from Great Britian (GB), France (FR), Italy (IT), and Bulgaria (BG). Relative risk for hepatitis B reactivation while undergoing Pembrolizumab treatment was statistically significant in the US and BG; (RR: 8.88, 95% CI: 3.00-26.31, P=0.0002) and (RR: 48.14, 95% CI: 7.37-314.29, P=0.0223), respectively. Data from other countries was statistically insignificant.
Discussion: Pembrolizumab functions as a potent check point inhibitor for various immune pathways. Immunotherapy as a class poses a risk of immunosuppression, which can result in hepatitis reactivation. Globally there were approximately 316 million people with hepatitis B in 2019. A staple of initiating immunotherapy is pretreatment screening. Despite pretreatment screening and antiviral therapy for hepatitis B, individuals undergoing immunotherapy develop hepatitis reactivation. Based on the results by country, the US and BG showed statistically significant, elevated RR for hepatitis B reactivation. Regardless of indication, individuals in US and BG undergoing pembrolizumab treatment have an 8.88- and 48.14-fold increased risk of hepatitis B reactivation when compared to individuals on other therapeutic treatment, respectively. This unexpected increased risk in the US and BG is likely due to reporting bias and small sample size. Hepatitis B reactivation is a rare adverse event in patients undergoing pembrolizumab therapy. There is a significant risk for reactivation in patients from the US and BG undergoing pembrolizumab treatment. The overall small sample size of reported events is due to effective pretreatment screening and treatment of hepatitis B.
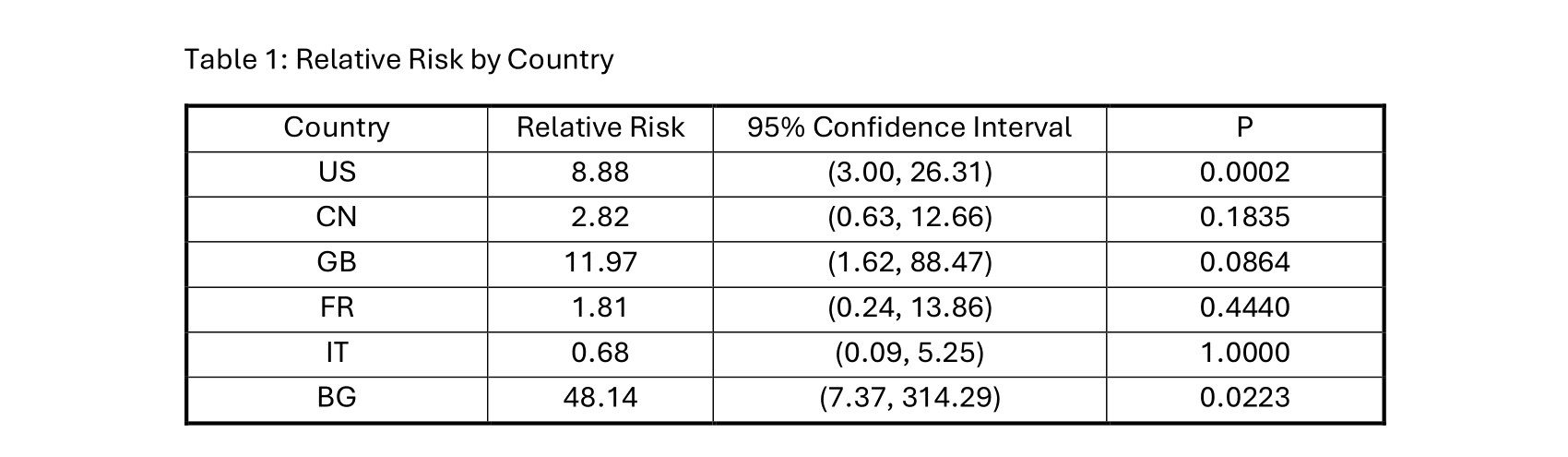
Figure: Table 1: Relative Risk by Country
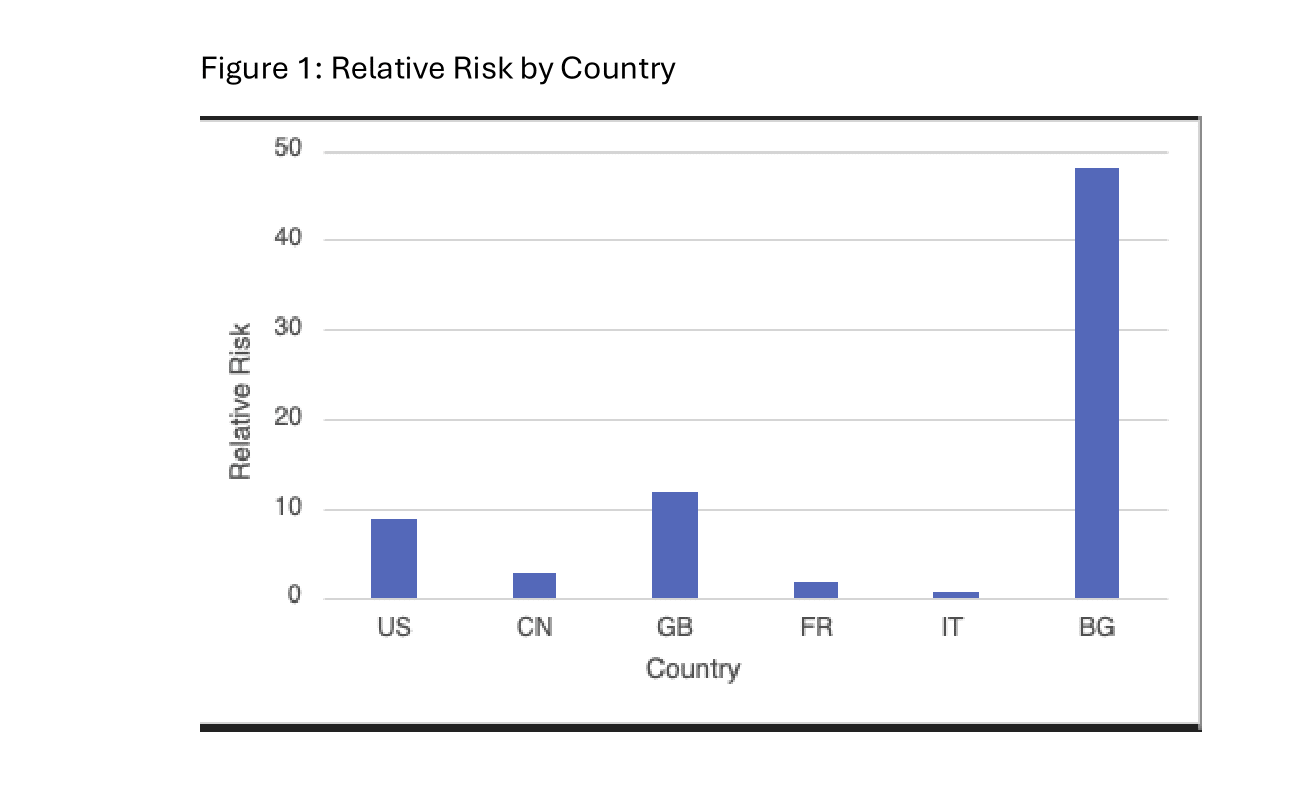
Figure: Figure 1: Relative Risk by Country
Disclosures:
Brian Li indicated no relevant financial relationships.
Abha Sathe indicated no relevant financial relationships.
Kabeer Ali indicated no relevant financial relationships.
Charles Harrison indicated no relevant financial relationships.
Brian Li, DO, Abha Sathe, MD, Kabeer Ali, MD, Charles J. Harrison, MD. P3677 - Retrospective Analysis of Hepatitis B Reactivation in Patients Undergoing Pembrolizumab Treatment by Country, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of Florida College of Medicine, Jacksonville, FL
Introduction: Our objective is to analyze the risk of hepatitis B reactivation in patients undergoing Pembrolizumab therapy based on country.
Methods: Data from the FDA Adverse Events Reporting System (FAERS) public dashboard was collected on hepatitis B reactivation in patients undergoing Pembrolizumab monotherapy. Data was also collected from FAERS to assess the total number of reported hepatitis B reactivations since 2014, when pembrolizumab was introduced. Both data sets were used to determine a relative risk for hepatitis B reactivation based on the reporting country.
Results: From 2014 to 2024, 3472 cases of hepatitis B reactivation were reported to the FAERS dashboard. 13 cases were reported in patients undergoing pembrolizumab treatment, approximately 0.37% of all cases. Of the 13 total cases, 7 (53.8%) were from the United States (US), 2 (15.4%) were from China (CN), and 1 each (7.7%) from Great Britian (GB), France (FR), Italy (IT), and Bulgaria (BG). Relative risk for hepatitis B reactivation while undergoing Pembrolizumab treatment was statistically significant in the US and BG; (RR: 8.88, 95% CI: 3.00-26.31, P=0.0002) and (RR: 48.14, 95% CI: 7.37-314.29, P=0.0223), respectively. Data from other countries was statistically insignificant.
Discussion: Pembrolizumab functions as a potent check point inhibitor for various immune pathways. Immunotherapy as a class poses a risk of immunosuppression, which can result in hepatitis reactivation. Globally there were approximately 316 million people with hepatitis B in 2019. A staple of initiating immunotherapy is pretreatment screening. Despite pretreatment screening and antiviral therapy for hepatitis B, individuals undergoing immunotherapy develop hepatitis reactivation. Based on the results by country, the US and BG showed statistically significant, elevated RR for hepatitis B reactivation. Regardless of indication, individuals in US and BG undergoing pembrolizumab treatment have an 8.88- and 48.14-fold increased risk of hepatitis B reactivation when compared to individuals on other therapeutic treatment, respectively. This unexpected increased risk in the US and BG is likely due to reporting bias and small sample size. Hepatitis B reactivation is a rare adverse event in patients undergoing pembrolizumab therapy. There is a significant risk for reactivation in patients from the US and BG undergoing pembrolizumab treatment. The overall small sample size of reported events is due to effective pretreatment screening and treatment of hepatitis B.

Figure: Table 1: Relative Risk by Country

Figure: Figure 1: Relative Risk by Country
Disclosures:
Brian Li indicated no relevant financial relationships.
Abha Sathe indicated no relevant financial relationships.
Kabeer Ali indicated no relevant financial relationships.
Charles Harrison indicated no relevant financial relationships.
Brian Li, DO, Abha Sathe, MD, Kabeer Ali, MD, Charles J. Harrison, MD. P3677 - Retrospective Analysis of Hepatitis B Reactivation in Patients Undergoing Pembrolizumab Treatment by Country, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
