Monday Poster Session
Category: Liver
P3675 - Improving Hepatocellular Carcinoma Surveillance in Veterans With Cirrhosis at the Miami Veterans Affairs Medical Center (MVAMC): A Quality Improvement (QI) Project
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Neelima Gaddipati, MD, MBA
University of Miami Miller School of Medicine at Jackson Memorial Hospital
Miami, FL
Presenting Author(s)
Neelima Gaddipati, MD, MBA1, Gema E. Casco, ARNP2, Alexander M. Sy, MD, MPH3, Binu John, MD, MPH, FACG4, Minh N. Hoang, MD2, Mina Shaker, MD, MSc5
1University of Miami Miller School of Medicine at Jackson Memorial Hospital, Miami, FL; 2Bruce W Carter Miami VA Medical Center, Miami, FL; 3Bruce W Carter Miami VA Medical Center / Herbert Wertheim College of Medicine Florida International University, Miami, FL; 4Miami VA and University of Miami, Miami, FL; 5Bruce W Carter Miami VA Medical Center, Herbert Wertheim College of Medicine Florida International University, and University of Miami Miller School of Medicine, Miami, FL
Introduction: Guidelines recommend hepatocellular carcinoma (HCC) surveillance in patients with cirrhosis via abdominal ultrasound with or without serum alpha-fetoprotein (AFP) every six months. In spite of this, studies have shown low surveillance rates, particularly in the United States, as surveillance rates are close to 17.8% nationally and 24.0% globally. Previous studies have performed targeted interventions at patient, specialist, primary care, and policy levels with varied results.
Methods: This QI initiative aimed to improve HCC surveillance rates so that 55% of Veterans with cirrhosis had appropriate abdominal imaging within the past six months by March 2025 and to 65% by March 2026. At the MVAMC, 634 Veterans had diagnoses of either cirrhosis and/or chronic Hepatitis B virus (HBV) in October 2024. 44.0% of Veterans were up to date with HCC surveillance, compared to 51.2% of Veterans nationally. The target population were identified via an Advanced Liver Disease VA National Dashboard. Via stakeholder interviews with MVAMC hepatologists, and institutional leaders, a fishbone diagram and driver diagram were created to identify areas for potential improvement. A chart review intervention was performed to place orders for abdominal ultrasound, AFP, and hepatology follow up as indicated (see Figure 1). If patients had inappropriate diagnoses of cirrhosis, cases were discussed with hepatologists prior to removing the diagnosis. The outcome measure was the percentage of Veterans with up-to-date imaging.
Results: The outcome measure was auto correlated. Data were graphed on a run chart, and visual analysis was used to evaluate for improvement (Figure 2). The median up-to-date surveillance rate at baseline in September and October 2024 was 45.1%. Post-intervention, this increased to 50.2%. A run in March 2025 demonstrated evidence of special cause variation, as ultrasounds ordered at the end of 2024 were being processed and completed. As a process measure, 160 charts were reviewed over six months. Incorrect cirrhosis diagnoses were identified and removed from 23 patients’ charts. Current screening rates are maintained around 52%.
Discussion: This intervention increased HCC surveillance rates and removed inappropriate cirrhosis diagnoses. Limitations include barriers to scheduling radiology appointments and dietary restrictions for ultrasounds, which will be addressed in the project’s next iteration.
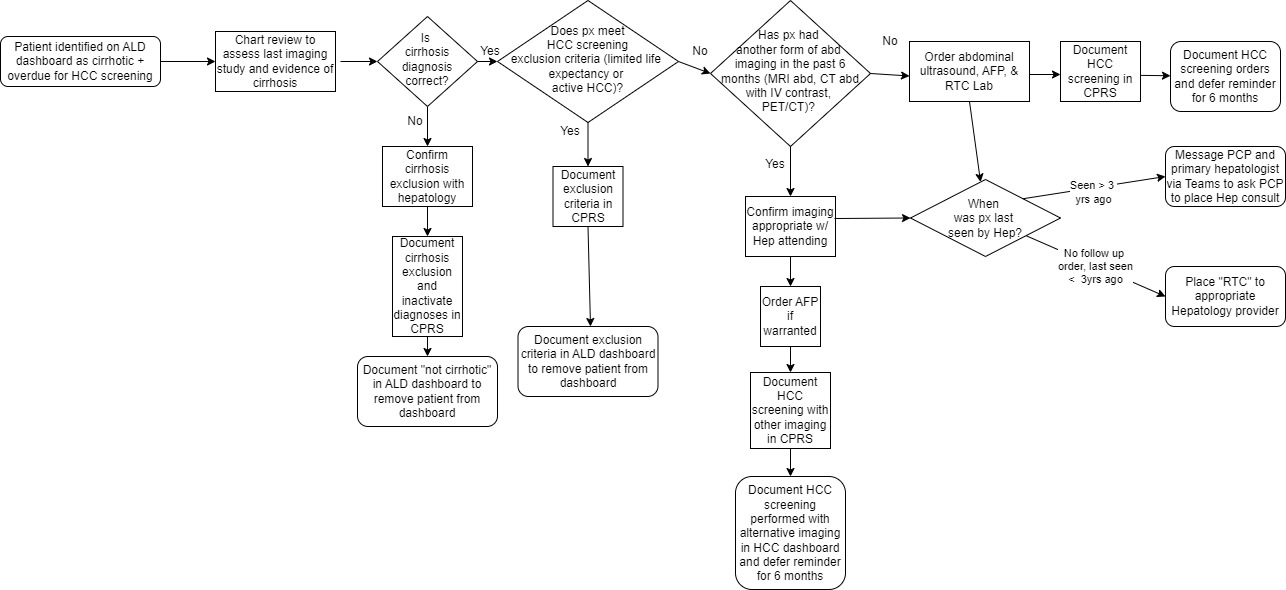
Figure: Figure 1: Process map depicting chart review intervention to evaluate appropriate candidates for HCC surveillance.
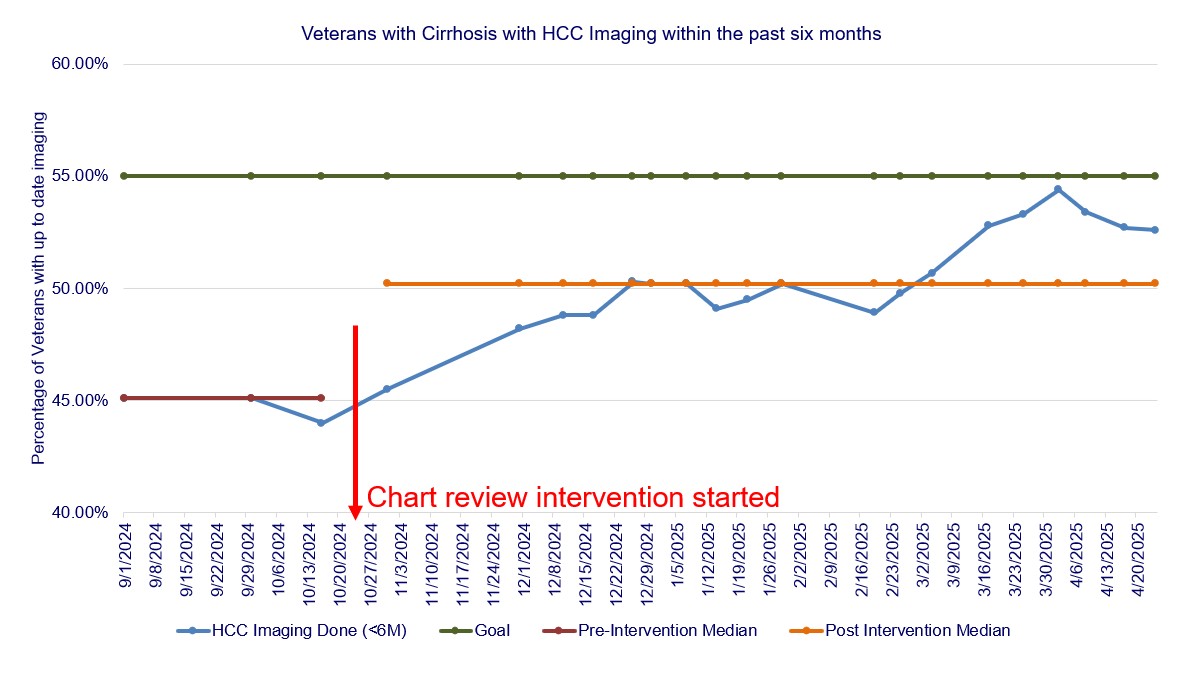
Figure: Figure 2: Run chart depicting pre- and post- intervention HCC surveillance rates. The median up-to-date surveillance rate at baseline in September and October 2024 was 45.1%. Post-intervention, this increased to 50.2%. A run in March 2025 demonstrated evidence of special cause variation, as ultrasounds ordered at the end of 2024 were being processed and completed.
Disclosures:
Neelima Gaddipati indicated no relevant financial relationships.
Gema Casco indicated no relevant financial relationships.
Alexander Sy indicated no relevant financial relationships.
Binu John: Exact sciences – Grant/Research Support. Genentech – Grant/Research Support. Gilead – Grant/Research Support. Glycotest, Inc – Grant/Research Support. Takeda – Grant/Research Support.
Minh Hoang indicated no relevant financial relationships.
Mina Shaker indicated no relevant financial relationships.
Neelima Gaddipati, MD, MBA1, Gema E. Casco, ARNP2, Alexander M. Sy, MD, MPH3, Binu John, MD, MPH, FACG4, Minh N. Hoang, MD2, Mina Shaker, MD, MSc5. P3675 - Improving Hepatocellular Carcinoma Surveillance in Veterans With Cirrhosis at the Miami Veterans Affairs Medical Center (MVAMC): A Quality Improvement (QI) Project, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Miami Miller School of Medicine at Jackson Memorial Hospital, Miami, FL; 2Bruce W Carter Miami VA Medical Center, Miami, FL; 3Bruce W Carter Miami VA Medical Center / Herbert Wertheim College of Medicine Florida International University, Miami, FL; 4Miami VA and University of Miami, Miami, FL; 5Bruce W Carter Miami VA Medical Center, Herbert Wertheim College of Medicine Florida International University, and University of Miami Miller School of Medicine, Miami, FL
Introduction: Guidelines recommend hepatocellular carcinoma (HCC) surveillance in patients with cirrhosis via abdominal ultrasound with or without serum alpha-fetoprotein (AFP) every six months. In spite of this, studies have shown low surveillance rates, particularly in the United States, as surveillance rates are close to 17.8% nationally and 24.0% globally. Previous studies have performed targeted interventions at patient, specialist, primary care, and policy levels with varied results.
Methods: This QI initiative aimed to improve HCC surveillance rates so that 55% of Veterans with cirrhosis had appropriate abdominal imaging within the past six months by March 2025 and to 65% by March 2026. At the MVAMC, 634 Veterans had diagnoses of either cirrhosis and/or chronic Hepatitis B virus (HBV) in October 2024. 44.0% of Veterans were up to date with HCC surveillance, compared to 51.2% of Veterans nationally. The target population were identified via an Advanced Liver Disease VA National Dashboard. Via stakeholder interviews with MVAMC hepatologists, and institutional leaders, a fishbone diagram and driver diagram were created to identify areas for potential improvement. A chart review intervention was performed to place orders for abdominal ultrasound, AFP, and hepatology follow up as indicated (see Figure 1). If patients had inappropriate diagnoses of cirrhosis, cases were discussed with hepatologists prior to removing the diagnosis. The outcome measure was the percentage of Veterans with up-to-date imaging.
Results: The outcome measure was auto correlated. Data were graphed on a run chart, and visual analysis was used to evaluate for improvement (Figure 2). The median up-to-date surveillance rate at baseline in September and October 2024 was 45.1%. Post-intervention, this increased to 50.2%. A run in March 2025 demonstrated evidence of special cause variation, as ultrasounds ordered at the end of 2024 were being processed and completed. As a process measure, 160 charts were reviewed over six months. Incorrect cirrhosis diagnoses were identified and removed from 23 patients’ charts. Current screening rates are maintained around 52%.
Discussion: This intervention increased HCC surveillance rates and removed inappropriate cirrhosis diagnoses. Limitations include barriers to scheduling radiology appointments and dietary restrictions for ultrasounds, which will be addressed in the project’s next iteration.

Figure: Figure 1: Process map depicting chart review intervention to evaluate appropriate candidates for HCC surveillance.

Figure: Figure 2: Run chart depicting pre- and post- intervention HCC surveillance rates. The median up-to-date surveillance rate at baseline in September and October 2024 was 45.1%. Post-intervention, this increased to 50.2%. A run in March 2025 demonstrated evidence of special cause variation, as ultrasounds ordered at the end of 2024 were being processed and completed.
Disclosures:
Neelima Gaddipati indicated no relevant financial relationships.
Gema Casco indicated no relevant financial relationships.
Alexander Sy indicated no relevant financial relationships.
Binu John: Exact sciences – Grant/Research Support. Genentech – Grant/Research Support. Gilead – Grant/Research Support. Glycotest, Inc – Grant/Research Support. Takeda – Grant/Research Support.
Minh Hoang indicated no relevant financial relationships.
Mina Shaker indicated no relevant financial relationships.
Neelima Gaddipati, MD, MBA1, Gema E. Casco, ARNP2, Alexander M. Sy, MD, MPH3, Binu John, MD, MPH, FACG4, Minh N. Hoang, MD2, Mina Shaker, MD, MSc5. P3675 - Improving Hepatocellular Carcinoma Surveillance in Veterans With Cirrhosis at the Miami Veterans Affairs Medical Center (MVAMC): A Quality Improvement (QI) Project, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
