Tuesday Poster Session
Category: Small Intestine
P6257 - Uncommon Territory: Isolated Duodenitis as a Manifestation of Checkpoint Inhibitor Toxicity
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Renieh M. Nabaty, MD
Henry Ford Health
Detroit, MI
Presenting Author(s)
Renieh M. Nabaty, MD, Polo Kostecki, DO, Najwa El-Nachef, MD
Henry Ford Health, Detroit, MI
Introduction: Pembrolizumab, a checkpoint inhibitor, is used in cancer immunotherapy and has improved survival outcomes in various malignancies. However, it is associated with immune-related adverse events, including gastrointestinal (GI) toxicity. While inflammatory changes in the GI tract—such as gastritis and colitis—are well-documented side effects, duodenitis remains poorly characterized and is largely described in case reports. Histologic findings of pembrolizumab-induced duodenitis may include villous blunting, increased lymphocytic and neutrophilic infiltration, and mucosal erosions, resembling celiac disease, in the absence of celiac serologies. We present a case of pembrolizumab-induced duodenitis.
Case Description/
Methods: A 67 year-old-male with metastatic squamous cell carcinoma of the lung undergoing treatment with pembrolizumab presented to the gastroenterology clinic with a 15-pound weight loss and diarrhea. He underwent an upper endoscopy 3 months after his immunotherapy was held that revealed concern for Barrett's esophagus as well as extensive nodularity in the duodenal mucosa (Figure 1). Duodenal pathology revealed prominent gastric foveolar metaplasia, focal villous shortening, and inflammation with reactive changes. He underwent a colonoscopy which did not reveal inflammation or ulceration. Random colonic biopsies were obtained to evaluate for colitis, however pathology was negative. Serologic testing for celiac disease was negative. Due to concern for immune checkpoint inhibitor-induced duodenitis and ongoing diarrheal symptoms, the patient started steroid therapy with taper. At a subsequent follow-up, the patient reported resolution of his diarrheal symptoms following steroid taper, along with weight gain.
Discussion: Duodenitis is an under-recognized manifestation of immune checkpoint inhibitors like pembrolizumab, often mimicking celiac disease. This may delay diagnosis and appropriate treatment with immunosuppressive therapy like corticosteroids. Given the increasing use of immunotherapy in oncology, raising awareness of atypical presentations, like pembrolizumab- induced duodenitis, is essential to improved patient outcomes. Thus, this case adds to the limited literature on this diagnosis and serves as a reminder to consider pembrolizumab-induced duodenitis on the differential.
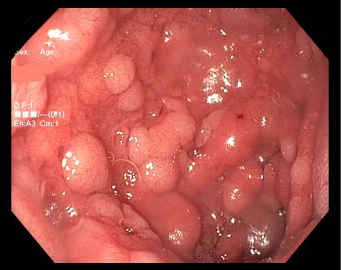
Figure: Nodular mucosa seen in the duodenum.
Disclosures:
Renieh Nabaty indicated no relevant financial relationships.
Polo Kostecki indicated no relevant financial relationships.
Najwa El-Nachef: Abbvie – Grant/Research Support. Abivax – Grant/Research Support. Genentech – Grant/Research Support. Takeda – Grant/Research Support.
Renieh M. Nabaty, MD, Polo Kostecki, DO, Najwa El-Nachef, MD. P6257 - Uncommon Territory: Isolated Duodenitis as a Manifestation of Checkpoint Inhibitor Toxicity, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Henry Ford Health, Detroit, MI
Introduction: Pembrolizumab, a checkpoint inhibitor, is used in cancer immunotherapy and has improved survival outcomes in various malignancies. However, it is associated with immune-related adverse events, including gastrointestinal (GI) toxicity. While inflammatory changes in the GI tract—such as gastritis and colitis—are well-documented side effects, duodenitis remains poorly characterized and is largely described in case reports. Histologic findings of pembrolizumab-induced duodenitis may include villous blunting, increased lymphocytic and neutrophilic infiltration, and mucosal erosions, resembling celiac disease, in the absence of celiac serologies. We present a case of pembrolizumab-induced duodenitis.
Case Description/
Methods: A 67 year-old-male with metastatic squamous cell carcinoma of the lung undergoing treatment with pembrolizumab presented to the gastroenterology clinic with a 15-pound weight loss and diarrhea. He underwent an upper endoscopy 3 months after his immunotherapy was held that revealed concern for Barrett's esophagus as well as extensive nodularity in the duodenal mucosa (Figure 1). Duodenal pathology revealed prominent gastric foveolar metaplasia, focal villous shortening, and inflammation with reactive changes. He underwent a colonoscopy which did not reveal inflammation or ulceration. Random colonic biopsies were obtained to evaluate for colitis, however pathology was negative. Serologic testing for celiac disease was negative. Due to concern for immune checkpoint inhibitor-induced duodenitis and ongoing diarrheal symptoms, the patient started steroid therapy with taper. At a subsequent follow-up, the patient reported resolution of his diarrheal symptoms following steroid taper, along with weight gain.
Discussion: Duodenitis is an under-recognized manifestation of immune checkpoint inhibitors like pembrolizumab, often mimicking celiac disease. This may delay diagnosis and appropriate treatment with immunosuppressive therapy like corticosteroids. Given the increasing use of immunotherapy in oncology, raising awareness of atypical presentations, like pembrolizumab- induced duodenitis, is essential to improved patient outcomes. Thus, this case adds to the limited literature on this diagnosis and serves as a reminder to consider pembrolizumab-induced duodenitis on the differential.

Figure: Nodular mucosa seen in the duodenum.
Disclosures:
Renieh Nabaty indicated no relevant financial relationships.
Polo Kostecki indicated no relevant financial relationships.
Najwa El-Nachef: Abbvie – Grant/Research Support. Abivax – Grant/Research Support. Genentech – Grant/Research Support. Takeda – Grant/Research Support.
Renieh M. Nabaty, MD, Polo Kostecki, DO, Najwa El-Nachef, MD. P6257 - Uncommon Territory: Isolated Duodenitis as a Manifestation of Checkpoint Inhibitor Toxicity, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
