Tuesday Poster Session
Category: Stomach and Spleen
P6315 - GLP-1 Receptor Agonist Use and Risk of Gastric Cancer: A Population-Based Retrospective Cohort Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- KA
Khaled Alsabbagh Alchirazi, MD
Aurora Health Care
Brookfield, WI
Presenting Author(s)
Muaz Alsabbagh, MD1, Khaled Alsabbagh Alchirazi, MD2, Kinan Obeidat, MD3, Moath Albliwi, MD4, Ahmed N.. Mohamed, MD5, Nour Azzouz, MD6, Nadia Huq, MD7
1Detroit Medical Center/Wayne State University, Cleveland, OH; 2Aurora Health Care, Brookfield, WI; 3University of Texas Medical Branch, Santa Fe, TX; 4Cleveland Clinic Foundation, Cleveland, OH; 5Cleveland Clinic Foundation, South Euclid, OH; 6University of Aleppo, Brookfield, WI; 7Advocate Aurora, Milwaukee, WI
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have emerged as a prominent class of medication due to their efficacy in managing obesity and related metabolic disorders. While beneficial metabolic and cardiovascular outcomes are well-documented, concerns remain regarding their long-term gastrointestinal safety, specifically the potential risk for gastric cancer. This study investigates the association between GLP-1RA exposure and gastric cancer incidence in a broad, real-world population.
Methods: We conducted a retrospective cohort study using the TriNetX electronic health record database. Adult patients without a prior diagnosis of gastric cancer were identified and categorized into two cohorts: patients receiving GLP-1RAs and matched controls who had never used GLP-1Ras, patients with gastric cancer prior to the index were excluded. Matching (1:1) was performed using propensity scores based on demographic characteristics and clinical risk factors, including obesity, Helicobacter pylori infection, alcohol use, personal hx of malignancy, family hx of neoplasm, BMI, gastritis, peptic ulcer disease, gastric ulcer and general comorbidities. The primary outcome was the incidence of gastric cancer, assessed via Cox proportional hazards models to calculate hazard ratios (HRs) with 95% confidence intervals (CIs).
Results: Post-matching cohorts (n=188,090 each) showed well-balanced characteristics. The mean current age was 60.4 years in the GLP-1RA group and 60.7 years in the control group. Female patients comprised 64.6% of GLP-1RA users and 65.3% of non-users, while males accounted for 30.9% and 30.6% and 17.5% and 18.6% were Black or African American, respectively. Most patients were White (65.0% vs. 65.3%). Clinical comorbidities such as obesity, diabetes, and GERD were similarly distributed between groups. Gastric cancer developed in 116 GLP-1RA users versus 290 non-users. GLP-1RA use was significantly associated with reduced gastric cancer risk (HR: 0.35; 95% CI, 0.28–0.44; p< 0.001).
Discussion: GLP-1RA therapy was associated with a significant reduction in gastric cancer risk in this large, matched cohort. These findings highlight potential gastrointestinal safety benefits of GLP-1RAs and suggest that, beyond their metabolic effects, these agents may offer protective effects against gastrointestinal malignancies. This observation warrants further exploration in mechanistic studies to better understand their role in cancer prevention and guide long-term prescribing practices.

Figure: Table1: General Characteristics Before and After Propensity Score Matching
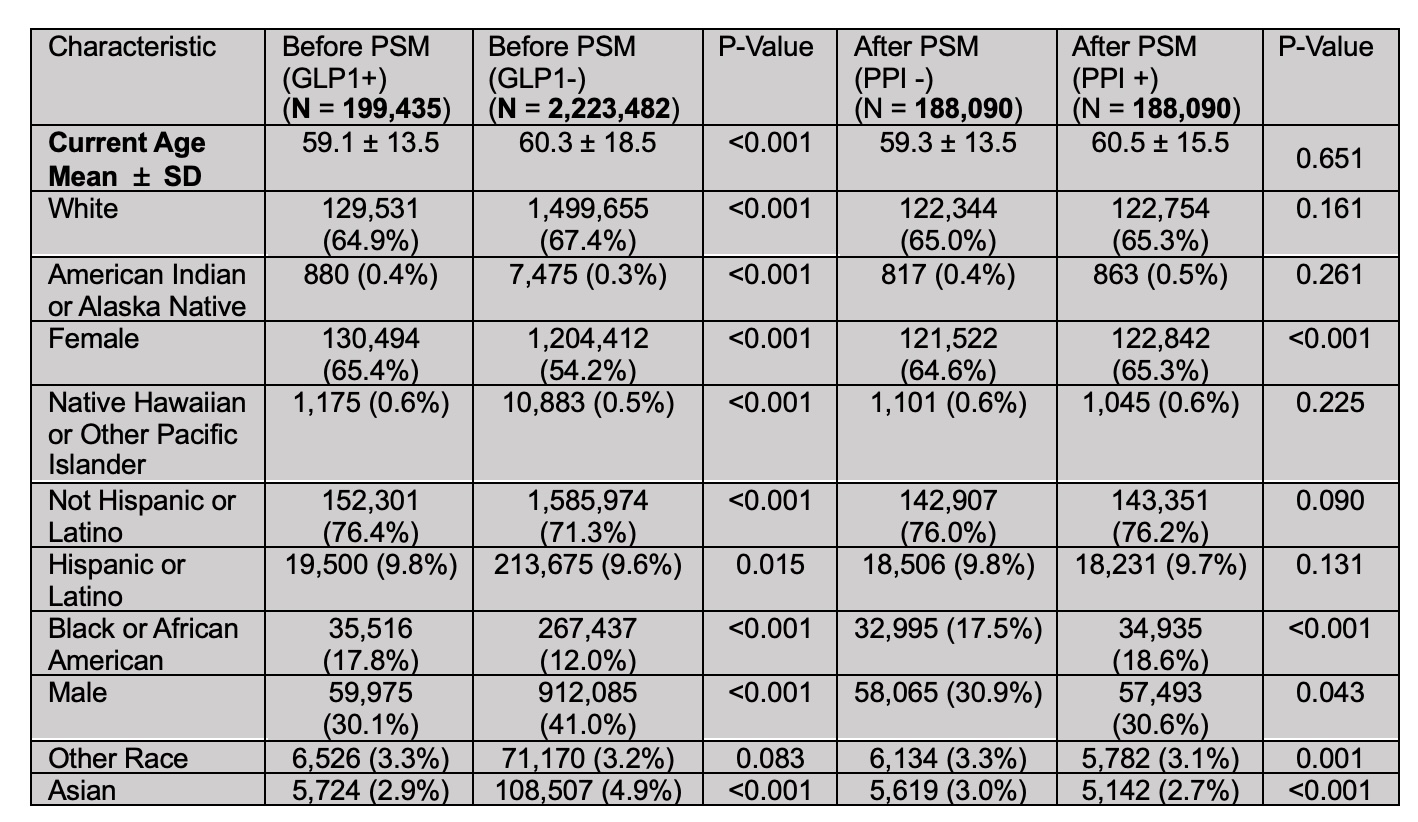
Figure: Table 2: Risks and Hazard Ratios (HRs) of First-Time Diagnosis of gastric cancer in patient receiving GLP1RA:
Disclosures:
Muaz Alsabbagh indicated no relevant financial relationships.
Khaled Alsabbagh Alchirazi indicated no relevant financial relationships.
Kinan Obeidat indicated no relevant financial relationships.
Moath Albliwi indicated no relevant financial relationships.
Ahmed Mohamed indicated no relevant financial relationships.
Nour Azzouz indicated no relevant financial relationships.
Nadia Huq indicated no relevant financial relationships.
Muaz Alsabbagh, MD1, Khaled Alsabbagh Alchirazi, MD2, Kinan Obeidat, MD3, Moath Albliwi, MD4, Ahmed N.. Mohamed, MD5, Nour Azzouz, MD6, Nadia Huq, MD7. P6315 - GLP-1 Receptor Agonist Use and Risk of Gastric Cancer: A Population-Based Retrospective Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Detroit Medical Center/Wayne State University, Cleveland, OH; 2Aurora Health Care, Brookfield, WI; 3University of Texas Medical Branch, Santa Fe, TX; 4Cleveland Clinic Foundation, Cleveland, OH; 5Cleveland Clinic Foundation, South Euclid, OH; 6University of Aleppo, Brookfield, WI; 7Advocate Aurora, Milwaukee, WI
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have emerged as a prominent class of medication due to their efficacy in managing obesity and related metabolic disorders. While beneficial metabolic and cardiovascular outcomes are well-documented, concerns remain regarding their long-term gastrointestinal safety, specifically the potential risk for gastric cancer. This study investigates the association between GLP-1RA exposure and gastric cancer incidence in a broad, real-world population.
Methods: We conducted a retrospective cohort study using the TriNetX electronic health record database. Adult patients without a prior diagnosis of gastric cancer were identified and categorized into two cohorts: patients receiving GLP-1RAs and matched controls who had never used GLP-1Ras, patients with gastric cancer prior to the index were excluded. Matching (1:1) was performed using propensity scores based on demographic characteristics and clinical risk factors, including obesity, Helicobacter pylori infection, alcohol use, personal hx of malignancy, family hx of neoplasm, BMI, gastritis, peptic ulcer disease, gastric ulcer and general comorbidities. The primary outcome was the incidence of gastric cancer, assessed via Cox proportional hazards models to calculate hazard ratios (HRs) with 95% confidence intervals (CIs).
Results: Post-matching cohorts (n=188,090 each) showed well-balanced characteristics. The mean current age was 60.4 years in the GLP-1RA group and 60.7 years in the control group. Female patients comprised 64.6% of GLP-1RA users and 65.3% of non-users, while males accounted for 30.9% and 30.6% and 17.5% and 18.6% were Black or African American, respectively. Most patients were White (65.0% vs. 65.3%). Clinical comorbidities such as obesity, diabetes, and GERD were similarly distributed between groups. Gastric cancer developed in 116 GLP-1RA users versus 290 non-users. GLP-1RA use was significantly associated with reduced gastric cancer risk (HR: 0.35; 95% CI, 0.28–0.44; p< 0.001).
Discussion: GLP-1RA therapy was associated with a significant reduction in gastric cancer risk in this large, matched cohort. These findings highlight potential gastrointestinal safety benefits of GLP-1RAs and suggest that, beyond their metabolic effects, these agents may offer protective effects against gastrointestinal malignancies. This observation warrants further exploration in mechanistic studies to better understand their role in cancer prevention and guide long-term prescribing practices.

Figure: Table1: General Characteristics Before and After Propensity Score Matching

Figure: Table 2: Risks and Hazard Ratios (HRs) of First-Time Diagnosis of gastric cancer in patient receiving GLP1RA:
Disclosures:
Muaz Alsabbagh indicated no relevant financial relationships.
Khaled Alsabbagh Alchirazi indicated no relevant financial relationships.
Kinan Obeidat indicated no relevant financial relationships.
Moath Albliwi indicated no relevant financial relationships.
Ahmed Mohamed indicated no relevant financial relationships.
Nour Azzouz indicated no relevant financial relationships.
Nadia Huq indicated no relevant financial relationships.
Muaz Alsabbagh, MD1, Khaled Alsabbagh Alchirazi, MD2, Kinan Obeidat, MD3, Moath Albliwi, MD4, Ahmed N.. Mohamed, MD5, Nour Azzouz, MD6, Nadia Huq, MD7. P6315 - GLP-1 Receptor Agonist Use and Risk of Gastric Cancer: A Population-Based Retrospective Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
