Tuesday Poster Session
Category: Liver
P6088 - A Case of Steroid-Refractory Immune Checkpoint Inhibitor Hepatitis Successfully Treated With Mycophenolate Mofetil
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Fawaz Hussain, MD
Wayne State University School of Medicine / Detroit Medical Center
Detroit, MI
Presenting Author(s)
Award: ACG Presidential Poster Award
Fawaz Hussain, MD1, Ahila Manivannan, MD1, Maryam Zaffer, BA2, Yusra F.. Shao, MD3, Karan Mathur, MD1
1Wayne State University School of Medicine / Detroit Medical Center, Detroit, MI; 2Nova Southeastern University, Clearwater, FL; 3Karmanos Cancer Institute, Detroit, MI
Introduction: Immune checkpoint inhibitors (ICI) have revolutionized cancer therapy, however they are associated with immune-related adverse events, including ICI hepatitis. While corticosteroids are first-line treatment, steroid-refractory cases pose a therapeutic challenge, with limited data guiding second-line immunosuppressive management. We describe a case of steroid-refractory grade 4 ICI hepatitis that responded successfully to mycophenolate mofetil (MMF).
Case Description/
Methods: A 36-year-old woman with metastatic melanoma presented with asymptomatic hepatocellular injury (ALT 346 U/L, AST 194 U/L) six weeks after her third cycle of nivolumab and ipilimumab. Despite ICI discontinuation and two weeks of oral prednisone at 1–1.5 mg/kg, her transaminases worsened. Her only medications included long-term bupropion 300 mg daily and magnesium supplementation. Serologic testing for all viral and autoimmune etiologies was negative. She was hospitalized and treated with IV pulse-dose steroids for three days, but her transaminases remained significantly elevated with peak ALT 1417 U/L and AST 327 U/L (Figure 1). Liver biopsy revealed CD8+ T-cell predominant infiltration, consistent with ICI-induced hepatocellular injury. MMF was initiated at 1 g BID, but was soon escalated to 1.5 g BID. Over the subsequent eight weeks, liver enzymes normalized and MMF was tapered over five weeks with a concurrent prednisone taper. There was sustained improvement in liver function without rebound elevation while off of immunosuppression. Her melanoma is currently under surveillance, with ongoing discussions regarding restarting immunotherapy.
Discussion: ICI hepatitis generally arises within 8 to 12 weeks of therapy and is driven by CD8+ T-cell-mediated hepatocellular injury. Evidence for second-line immunosuppressants is limited. Current clinical practice is largely based on case reports with minimal information on dosing and duration of these agents. This case supports the use of high-dose MMF as an effective second-line immunosuppressive agent in ICI hepatitis. It also highlights that prolonged high-dose steroid therapy was ineffective at markedly elevated liver enzyme levels, and earlier initiation of second-line therapy could have been beneficial. These patients may require prolonged immunosuppression to prevent rebound hepatitis and manage other immune toxicities. Reintroducing immunotherapy after ICI hepatitis also remains a challenge and must be individualized.
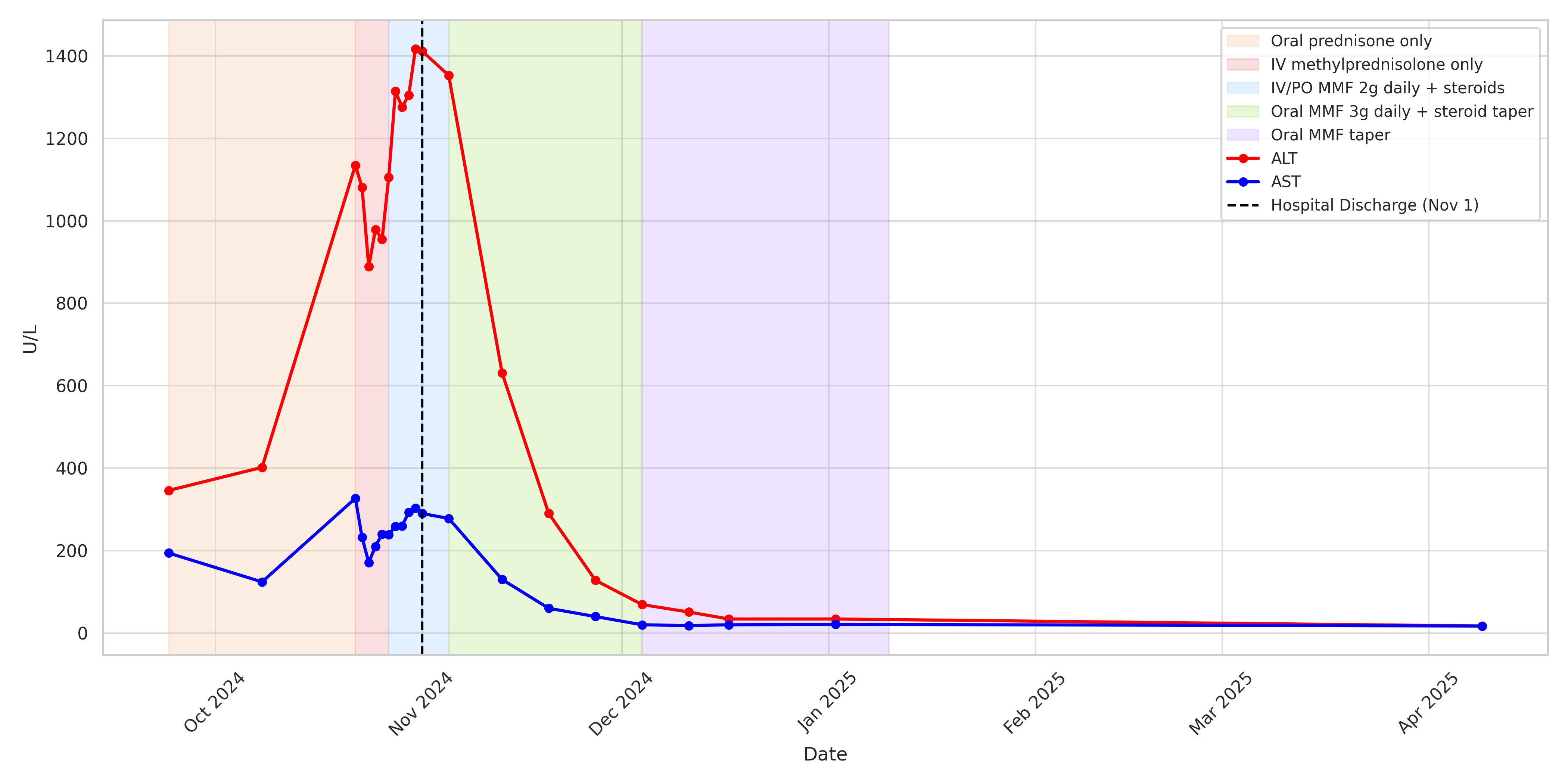
Figure: Liver function test (LFT) trends during treatment of steroid-refractory immune checkpoint inhibitor (ICI) hepatitis.
AST and ALT levels trended over time throughout the treatment course. The shaded background highlights five distinct immunosuppressive treatment phases: (1) oral prednisone monotherapy, (2) intravenous methylprednisolone monotherapy, (3) addition of IV mycophenolate mofetil (MMF) 2 g total daily with corticosteroids; transitioned to PO upon discharge, (4) increasing MMF 3 g total daily with corticosteroid taper, and (5) MMF taper 500 mg each week until discontinuation. Progressive improvement in transaminase levels was noted following MMF escalation and steroid taper.
Disclosures:
Fawaz Hussain indicated no relevant financial relationships.
Ahila Manivannan indicated no relevant financial relationships.
Maryam Zaffer indicated no relevant financial relationships.
Yusra Shao indicated no relevant financial relationships.
Karan Mathur indicated no relevant financial relationships.
Fawaz Hussain, MD1, Ahila Manivannan, MD1, Maryam Zaffer, BA2, Yusra F.. Shao, MD3, Karan Mathur, MD1. P6088 - A Case of Steroid-Refractory Immune Checkpoint Inhibitor Hepatitis Successfully Treated With Mycophenolate Mofetil, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Fawaz Hussain, MD1, Ahila Manivannan, MD1, Maryam Zaffer, BA2, Yusra F.. Shao, MD3, Karan Mathur, MD1
1Wayne State University School of Medicine / Detroit Medical Center, Detroit, MI; 2Nova Southeastern University, Clearwater, FL; 3Karmanos Cancer Institute, Detroit, MI
Introduction: Immune checkpoint inhibitors (ICI) have revolutionized cancer therapy, however they are associated with immune-related adverse events, including ICI hepatitis. While corticosteroids are first-line treatment, steroid-refractory cases pose a therapeutic challenge, with limited data guiding second-line immunosuppressive management. We describe a case of steroid-refractory grade 4 ICI hepatitis that responded successfully to mycophenolate mofetil (MMF).
Case Description/
Methods: A 36-year-old woman with metastatic melanoma presented with asymptomatic hepatocellular injury (ALT 346 U/L, AST 194 U/L) six weeks after her third cycle of nivolumab and ipilimumab. Despite ICI discontinuation and two weeks of oral prednisone at 1–1.5 mg/kg, her transaminases worsened. Her only medications included long-term bupropion 300 mg daily and magnesium supplementation. Serologic testing for all viral and autoimmune etiologies was negative. She was hospitalized and treated with IV pulse-dose steroids for three days, but her transaminases remained significantly elevated with peak ALT 1417 U/L and AST 327 U/L (Figure 1). Liver biopsy revealed CD8+ T-cell predominant infiltration, consistent with ICI-induced hepatocellular injury. MMF was initiated at 1 g BID, but was soon escalated to 1.5 g BID. Over the subsequent eight weeks, liver enzymes normalized and MMF was tapered over five weeks with a concurrent prednisone taper. There was sustained improvement in liver function without rebound elevation while off of immunosuppression. Her melanoma is currently under surveillance, with ongoing discussions regarding restarting immunotherapy.
Discussion: ICI hepatitis generally arises within 8 to 12 weeks of therapy and is driven by CD8+ T-cell-mediated hepatocellular injury. Evidence for second-line immunosuppressants is limited. Current clinical practice is largely based on case reports with minimal information on dosing and duration of these agents. This case supports the use of high-dose MMF as an effective second-line immunosuppressive agent in ICI hepatitis. It also highlights that prolonged high-dose steroid therapy was ineffective at markedly elevated liver enzyme levels, and earlier initiation of second-line therapy could have been beneficial. These patients may require prolonged immunosuppression to prevent rebound hepatitis and manage other immune toxicities. Reintroducing immunotherapy after ICI hepatitis also remains a challenge and must be individualized.

Figure: Liver function test (LFT) trends during treatment of steroid-refractory immune checkpoint inhibitor (ICI) hepatitis.
AST and ALT levels trended over time throughout the treatment course. The shaded background highlights five distinct immunosuppressive treatment phases: (1) oral prednisone monotherapy, (2) intravenous methylprednisolone monotherapy, (3) addition of IV mycophenolate mofetil (MMF) 2 g total daily with corticosteroids; transitioned to PO upon discharge, (4) increasing MMF 3 g total daily with corticosteroid taper, and (5) MMF taper 500 mg each week until discontinuation. Progressive improvement in transaminase levels was noted following MMF escalation and steroid taper.
Disclosures:
Fawaz Hussain indicated no relevant financial relationships.
Ahila Manivannan indicated no relevant financial relationships.
Maryam Zaffer indicated no relevant financial relationships.
Yusra Shao indicated no relevant financial relationships.
Karan Mathur indicated no relevant financial relationships.
Fawaz Hussain, MD1, Ahila Manivannan, MD1, Maryam Zaffer, BA2, Yusra F.. Shao, MD3, Karan Mathur, MD1. P6088 - A Case of Steroid-Refractory Immune Checkpoint Inhibitor Hepatitis Successfully Treated With Mycophenolate Mofetil, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

