Tuesday Poster Session
Category: Liver
P6070 - Challenging the Dogma: Reversal of End-Stage Liver Fibrosis With Tirzepatide in MASH Cirrhosis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- TN
Thuy-Duyen Nguyen, MD
Mayo Clinic
Phoenix, AZ
Presenting Author(s)
Thuy-Duyen Nguyen, MD, Dora Lam-Himlin, MD, Lizaola-Mayo Blanca, MD, David Chascsa, MD
Mayo Clinic, Phoenix, AZ
Introduction: Rising metabolic-associated steatotic liver disease (MASLD)/metabolic-associated steatohepatitis (MASH) prevalence is forecasted to reach 55% by 2040, emphasizing the demand for effective therapies. While resmetirom is the first approved treatment for MASH, the landscape is evolving with more agents being investigated, like dual glucagon-like peptide-1 receptor (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) agonists. Notably, Loomba et al.'s (2024) tirzepatide study showed resolving inflammation without worsening fibrosis. However, these therapies, including resmetirom, restrict use to patients with stage F2-F3 due to cirrhosis decompensation risks, necessitating careful consideration in this population.
Case Description/
Methods: A 46 y.o. obese female with decompensated cirrhosis from MASH and alcohol underwent a deceased-donor liver transplant in 2019. Within the first year post-transplant, she gained 36kg and exhibited evidence of hepatic steatosis. Her FibroSCAN revealed CAP of 400dB/M (S3 steatosis) and LSM of 61.2kPA (F4 fibrosis). Follow-up biopsy confirmed findings of severe steatohepatitis (NAS 7/8) and F3 fibrosis, attributed to metabolic dysfunction without evidence of alcohol recurrence. She decompensated with ascites and varices, leading to transplant re-listing at MELD 29 in 2020. Despite 2 years of intensive lifestyle modification, losing 17kg, and recompensation, her follow-up FibroSCAN showed persistent S3 and F4, and biopsy revealed progression to cirrhosis with bridging fibrosis and ongoing steatohepatitis.
Steroid use for immunosuppression led to her developing type 2 diabetes, and subsequently, tirzepatide was initiated in 2023, resulting in a 13.6kg loss. Despite her 2024 FibroSCAN showing persistent S3, her LSM improved to 10.3kPa (F3), hinting at possible fibrosis regression. Over the next year, she lost another 29.5kg despite a tirzepatide dose reduction. Shockingly, her 2025 FibroSCAN revealed further fibrosis regression with LSM 5.5kPa (F1) and steatohepatitis resolution (CAP 204dB/m, S0). A liver biopsy confirmed fibrosis regression to F2-F3 and steatohepatitis resolution.
Discussion: This case challenges the widely accepted dogma that liver scarring is irreversible at stage F4 with multiple liver fibrosis monitoring modalities demonstrate fibrosis reversal, including serologies, FibroSCAN, and biopsy. This suggests that GLP-1/GLP agonists may be safe for cirrhotic patients and halt further fibrosis or promote reversal.
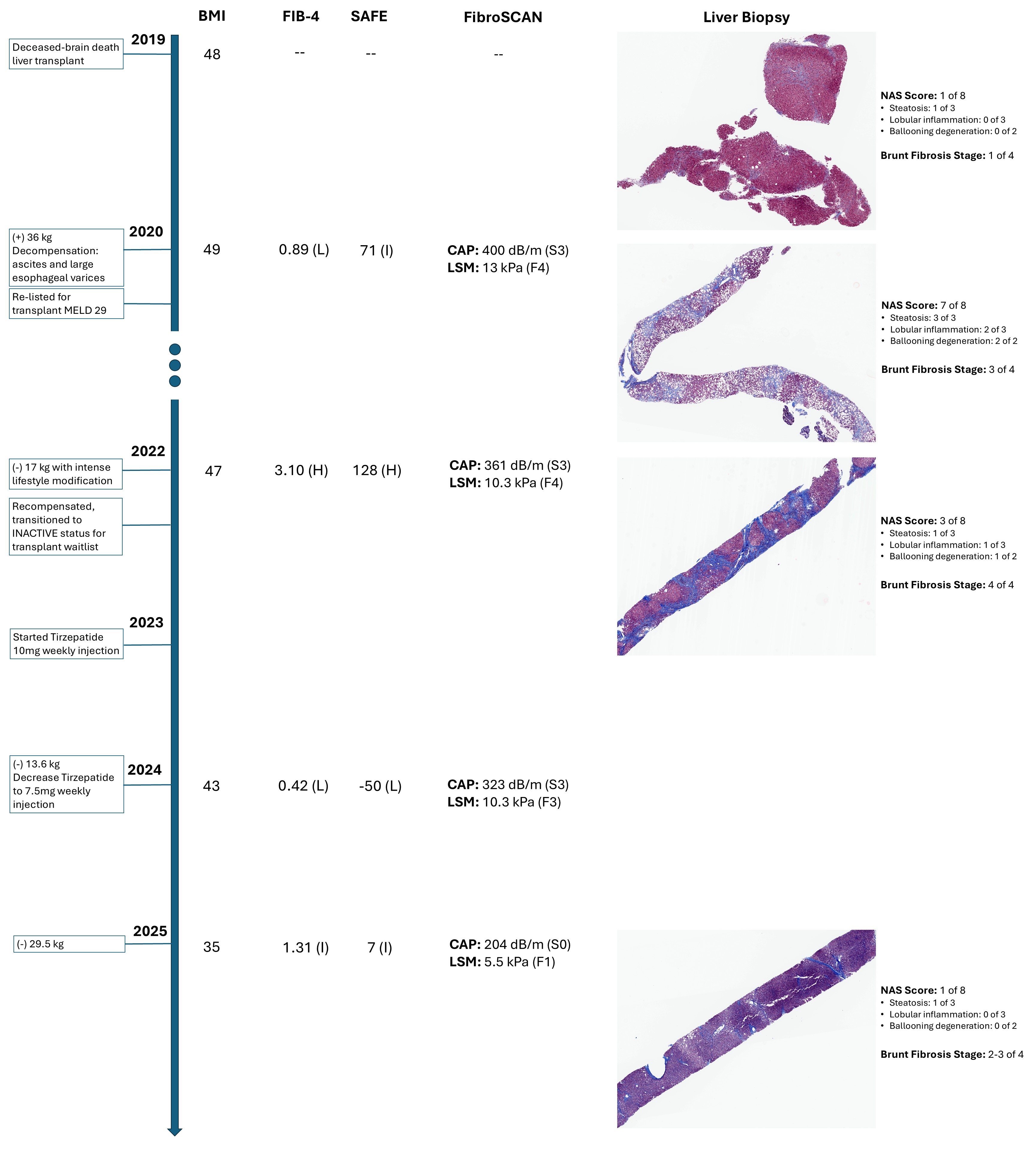
Figure: Timeline of patient events with corresponding BMI, non-invasive fibrosis evaluators (FIB-4, SAFE score, and FibroSCAN), and liver biopsies. Biopsy at time of transplant demonstrated low steatosis (NAS 1/8) and F1 fibrosis. Following a 36 kg weight gain, her 2020 biopsy showed a concerning increase in steatosis (NAS 7/8) and fibrosis progression to F3. While 17 kg of weight loss through lifestyle modification by 2022 improved steatosis (NAS 3/8), fibrosis unfortunately advanced to stage 4. The introduction of tirzepatide in 2023 led to a substantial 43.1 kg weight reduction, and follow-up 2025 biopsy revealed full resolution of steatosis (NAS 1/8) and significant regression of fibrosis from stage 4 to 2-3.
Disclosures:
Thuy-Duyen Nguyen indicated no relevant financial relationships.
Dora Lam-Himlin indicated no relevant financial relationships.
Lizaola-Mayo Blanca indicated no relevant financial relationships.
David Chascsa indicated no relevant financial relationships.
Thuy-Duyen Nguyen, MD, Dora Lam-Himlin, MD, Lizaola-Mayo Blanca, MD, David Chascsa, MD. P6070 - Challenging the Dogma: Reversal of End-Stage Liver Fibrosis With Tirzepatide in MASH Cirrhosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Mayo Clinic, Phoenix, AZ
Introduction: Rising metabolic-associated steatotic liver disease (MASLD)/metabolic-associated steatohepatitis (MASH) prevalence is forecasted to reach 55% by 2040, emphasizing the demand for effective therapies. While resmetirom is the first approved treatment for MASH, the landscape is evolving with more agents being investigated, like dual glucagon-like peptide-1 receptor (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) agonists. Notably, Loomba et al.'s (2024) tirzepatide study showed resolving inflammation without worsening fibrosis. However, these therapies, including resmetirom, restrict use to patients with stage F2-F3 due to cirrhosis decompensation risks, necessitating careful consideration in this population.
Case Description/
Methods: A 46 y.o. obese female with decompensated cirrhosis from MASH and alcohol underwent a deceased-donor liver transplant in 2019. Within the first year post-transplant, she gained 36kg and exhibited evidence of hepatic steatosis. Her FibroSCAN revealed CAP of 400dB/M (S3 steatosis) and LSM of 61.2kPA (F4 fibrosis). Follow-up biopsy confirmed findings of severe steatohepatitis (NAS 7/8) and F3 fibrosis, attributed to metabolic dysfunction without evidence of alcohol recurrence. She decompensated with ascites and varices, leading to transplant re-listing at MELD 29 in 2020. Despite 2 years of intensive lifestyle modification, losing 17kg, and recompensation, her follow-up FibroSCAN showed persistent S3 and F4, and biopsy revealed progression to cirrhosis with bridging fibrosis and ongoing steatohepatitis.
Steroid use for immunosuppression led to her developing type 2 diabetes, and subsequently, tirzepatide was initiated in 2023, resulting in a 13.6kg loss. Despite her 2024 FibroSCAN showing persistent S3, her LSM improved to 10.3kPa (F3), hinting at possible fibrosis regression. Over the next year, she lost another 29.5kg despite a tirzepatide dose reduction. Shockingly, her 2025 FibroSCAN revealed further fibrosis regression with LSM 5.5kPa (F1) and steatohepatitis resolution (CAP 204dB/m, S0). A liver biopsy confirmed fibrosis regression to F2-F3 and steatohepatitis resolution.
Discussion: This case challenges the widely accepted dogma that liver scarring is irreversible at stage F4 with multiple liver fibrosis monitoring modalities demonstrate fibrosis reversal, including serologies, FibroSCAN, and biopsy. This suggests that GLP-1/GLP agonists may be safe for cirrhotic patients and halt further fibrosis or promote reversal.

Figure: Timeline of patient events with corresponding BMI, non-invasive fibrosis evaluators (FIB-4, SAFE score, and FibroSCAN), and liver biopsies. Biopsy at time of transplant demonstrated low steatosis (NAS 1/8) and F1 fibrosis. Following a 36 kg weight gain, her 2020 biopsy showed a concerning increase in steatosis (NAS 7/8) and fibrosis progression to F3. While 17 kg of weight loss through lifestyle modification by 2022 improved steatosis (NAS 3/8), fibrosis unfortunately advanced to stage 4. The introduction of tirzepatide in 2023 led to a substantial 43.1 kg weight reduction, and follow-up 2025 biopsy revealed full resolution of steatosis (NAS 1/8) and significant regression of fibrosis from stage 4 to 2-3.
Disclosures:
Thuy-Duyen Nguyen indicated no relevant financial relationships.
Dora Lam-Himlin indicated no relevant financial relationships.
Lizaola-Mayo Blanca indicated no relevant financial relationships.
David Chascsa indicated no relevant financial relationships.
Thuy-Duyen Nguyen, MD, Dora Lam-Himlin, MD, Lizaola-Mayo Blanca, MD, David Chascsa, MD. P6070 - Challenging the Dogma: Reversal of End-Stage Liver Fibrosis With Tirzepatide in MASH Cirrhosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
