Tuesday Poster Session
Category: Liver
P5996 - Drug-Induced Liver Injury From Off-Label Fenbendazole Use in Metastatic Breast Cancer: A Case Report
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Muskaan Chaturvedi, BS (she/her/hers)
University of Texas Southwestern Medical Center
Dallas, TX
Presenting Author(s)
Muskaan Chaturvedi, BS, Ludovic Saba, MD, MSc, Azraa Amroze, MD, Jingwen Zhang, MD, Michael McConville, PhD, Grace Selner, BS, Jaini Sutaria, MBBS, MD
University of Texas Southwestern Medical Center, Dallas, TX
Introduction: Drug-induced liver injury (DILI) is a diagnostic challenge, especially when linked to unregulated or repurposed agents. We present a case of DILI in a patient with metastatic breast cancer, likely due to self-administration of fenbendazole -- a benzimidazole anthelmintic approved only for veterinary use. Although preclinical data suggests antiproliferative effects, rigorous human data are lacking. Despite no FDA approval, its use among cancer patients is rising, fueled by anecdotal reports on social media. To date, only two published case reports describe severe fenbendazole-associated hepatocellular DILI, both resolving after drug cessation.
Case Description/
Methods: A 49-year-old woman with newly diagnosed ER-/PR-/HER2+ grade 3 invasive ductal carcinoma of the left breast with axillary nodal involvement presented for routine pre-chemotherapy workup. She was found to have markedly elevated liver enzymes: AST 679, ALT 1119, Alk Phos 122, TBili 0.5 (up from one week prior: AST 258, ALT 402, Alk Phos 106, TBili 0.4). She was asymptomatic and denied recent illnesses, travel, and alcohol, illicit drug, and supplement use, reporting only taking acetaminophen 500 mg daily for pain. Her son later disclosed she had been taking veterinary-grade fenbendazole orally 4 days per week for 4-6 weeks, influenced by social media posts promoting its anticancer and analgesic benefits.
Workup showed negative hepatitis A, B, and C serologies, acetaminophen level < 5 µg/mL, ANA 1:320, PT and INR at 14.0 and 1.3, and no signs of hepatic encephalopathy. Abdominal ultrasound showed mild hepatic steatosis without biliary dilatation; contrast CT abdomen/pelvis revealed no hepatic metastases or obstruction. Hepatology was consulted for presumed DILI and N-acetylcysteine was empirically initiated to aid with liver function recovery. By day 4, liver enzymes improved (AST 545, ALT 874, Alk Phos 93, TBili 0.6), and the patient remained clinically stable. She was discharged with counseling to discontinue fenbendazole and to present for outpatient LFT monitoring and oncology follow up.
Discussion: Fenbendazole, though intended for veterinary use, poses a real risk for hepatocellular DILI when misused in humans. This case, alongside previous reports, highlights the need for routine screening of non-prescribed agents, especially those promoted online. Early recognition, drug withdrawal, and supportive care can lead to prompt recovery. However, systematic safety evaluation and patient education are urgently needed.

Figure: The medication the patient was taking, image provided by her son. Fenbendazole is easily available over the counter as canine dewormer.
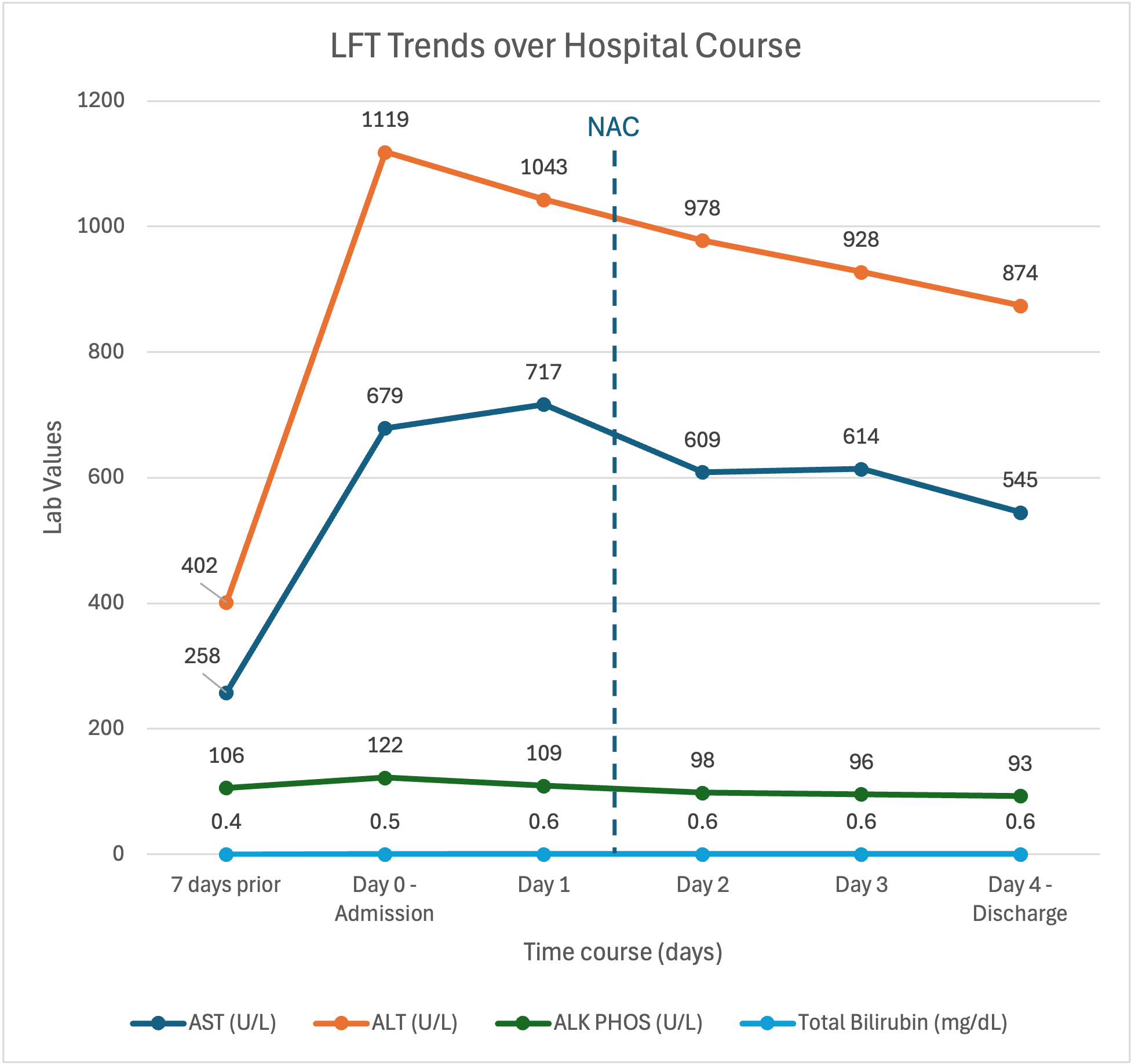
Figure: LFTs trended over patient's hospital course.
Disclosures:
Muskaan Chaturvedi indicated no relevant financial relationships.
Ludovic Saba indicated no relevant financial relationships.
Azraa Amroze indicated no relevant financial relationships.
Jingwen Zhang indicated no relevant financial relationships.
Michael McConville indicated no relevant financial relationships.
Grace Selner indicated no relevant financial relationships.
Jaini Sutaria indicated no relevant financial relationships.
Muskaan Chaturvedi, BS, Ludovic Saba, MD, MSc, Azraa Amroze, MD, Jingwen Zhang, MD, Michael McConville, PhD, Grace Selner, BS, Jaini Sutaria, MBBS, MD. P5996 - Drug-Induced Liver Injury From Off-Label Fenbendazole Use in Metastatic Breast Cancer: A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of Texas Southwestern Medical Center, Dallas, TX
Introduction: Drug-induced liver injury (DILI) is a diagnostic challenge, especially when linked to unregulated or repurposed agents. We present a case of DILI in a patient with metastatic breast cancer, likely due to self-administration of fenbendazole -- a benzimidazole anthelmintic approved only for veterinary use. Although preclinical data suggests antiproliferative effects, rigorous human data are lacking. Despite no FDA approval, its use among cancer patients is rising, fueled by anecdotal reports on social media. To date, only two published case reports describe severe fenbendazole-associated hepatocellular DILI, both resolving after drug cessation.
Case Description/
Methods: A 49-year-old woman with newly diagnosed ER-/PR-/HER2+ grade 3 invasive ductal carcinoma of the left breast with axillary nodal involvement presented for routine pre-chemotherapy workup. She was found to have markedly elevated liver enzymes: AST 679, ALT 1119, Alk Phos 122, TBili 0.5 (up from one week prior: AST 258, ALT 402, Alk Phos 106, TBili 0.4). She was asymptomatic and denied recent illnesses, travel, and alcohol, illicit drug, and supplement use, reporting only taking acetaminophen 500 mg daily for pain. Her son later disclosed she had been taking veterinary-grade fenbendazole orally 4 days per week for 4-6 weeks, influenced by social media posts promoting its anticancer and analgesic benefits.
Workup showed negative hepatitis A, B, and C serologies, acetaminophen level < 5 µg/mL, ANA 1:320, PT and INR at 14.0 and 1.3, and no signs of hepatic encephalopathy. Abdominal ultrasound showed mild hepatic steatosis without biliary dilatation; contrast CT abdomen/pelvis revealed no hepatic metastases or obstruction. Hepatology was consulted for presumed DILI and N-acetylcysteine was empirically initiated to aid with liver function recovery. By day 4, liver enzymes improved (AST 545, ALT 874, Alk Phos 93, TBili 0.6), and the patient remained clinically stable. She was discharged with counseling to discontinue fenbendazole and to present for outpatient LFT monitoring and oncology follow up.
Discussion: Fenbendazole, though intended for veterinary use, poses a real risk for hepatocellular DILI when misused in humans. This case, alongside previous reports, highlights the need for routine screening of non-prescribed agents, especially those promoted online. Early recognition, drug withdrawal, and supportive care can lead to prompt recovery. However, systematic safety evaluation and patient education are urgently needed.

Figure: The medication the patient was taking, image provided by her son. Fenbendazole is easily available over the counter as canine dewormer.

Figure: LFTs trended over patient's hospital course.
Disclosures:
Muskaan Chaturvedi indicated no relevant financial relationships.
Ludovic Saba indicated no relevant financial relationships.
Azraa Amroze indicated no relevant financial relationships.
Jingwen Zhang indicated no relevant financial relationships.
Michael McConville indicated no relevant financial relationships.
Grace Selner indicated no relevant financial relationships.
Jaini Sutaria indicated no relevant financial relationships.
Muskaan Chaturvedi, BS, Ludovic Saba, MD, MSc, Azraa Amroze, MD, Jingwen Zhang, MD, Michael McConville, PhD, Grace Selner, BS, Jaini Sutaria, MBBS, MD. P5996 - Drug-Induced Liver Injury From Off-Label Fenbendazole Use in Metastatic Breast Cancer: A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

