Monday Poster Session
Category: Liver
P3701 - The Impact of Systemic Chemoimmunotherapy in Combination With Locoregional Therapies on Transplant Rejection in Liver Transplant Secondary to Hepatocellular Carcinoma (HCC)
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- AK
Ali Khurram, MD
Dallas Methodist
Dallas, TX
Presenting Author(s)
Award: ACG Presidential Poster Award
Ali Khurram, MD1, Khadyoth Nanneboyina, MD1, Parvez Mantry, MD, CPE2, Ashwini Mehta, DO1, Judith Pozzerle, RN1
1Dallas Methodist, Dallas, TX; 2Methodist Dallas Medical Center, Dallas, TX
Introduction: Liver transplantation (LT) is the gold standard treatment for unresectable HCC. However, many patients with HCC are outside of the Milan criteria for LT eligibility. In such cases, downstaging therapies decrease tumor burden to increase eligibility. Traditional downstaging methods include locoregional therapy (LRT) but recently, systemic therapy, particularly immune checkpoint inhibitors (ICIs), have shown promise. The use of ICIs in the treatment of HCC raises the concern for increased possibility of rejection following LT.
Methods: This study was a single-center retrospective outcome analysis of all patients diagnosed with HCC who underwent LT between June 2018 and March 2024 (n = 93) with primary endpoints being post-LT rejection and Rejection Activity Index (RAI) grade. Patients were categorized into 2 groups: 1) LRT alone and 2) combination of LRT and systemic therapy. LRT included Transarterial chemoembolization, Radioembolization, Microwave Ablation, and Stereotactic Beam Radiation Therapy. Systemic therapies included Nivolumab + Ipilimumab, Atezolizumab + Bevacizumab, Sorafenib, Lenvatinib, Ramuricumab, and Cabozantinib. T-test was performed.
Results: 78 patients received LRT alone and 15 patients received combination therapy. Median total tumor diameter on MRI prior to treatment initiation of the LRT group was 3 cm vs. 4.6 cm in the combination group (p = 0.02). Median alpha-fetoprotein (AFP) prior to treatment initiation of the LRT group was 5.4 ng/mL vs. 12.3 ng/mL in the combination group (p = 0.004). Within the combination group, 2 patients received Nivolumab + Ipilimumab, 7 received Atezolizumab + Bevacizumab, 6 received Sorafenib, 3 received Lenvatinib, 1 received Ramucirumab, and 2 received Cabozantinib. The incidence of transplant rejection in the LRT group was 22.6% vs. 20% in combination group (p = 0.43). The median RAI grade of rejection episodes in the LRT group was 5 vs. 6 in the combination group (p = 0.44). The incidence of serious infections requiring hospitalization in the LRT group was 15.4% vs. 6.7% in the combination group (p = 0.11).
Discussion: Our results show that combination therapy for the treatment of HCC was associated with similar rates of transplant rejection, RAI grade of rejection, and serious infections requiring hospitalization compared to patients who received LRT only. This preliminary data suggests that systemic therapies, such as ICIs, are safe to use for downstaging of HCC to potentially improve outcomes and increase transplant eligibility.
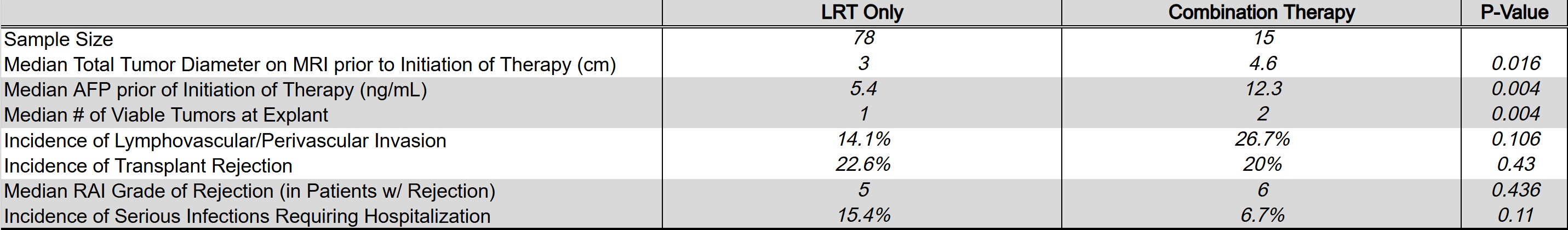
Figure: Comparison of LRT and combination patients
Disclosures:
Ali Khurram indicated no relevant financial relationships.
Khadyoth Nanneboyina indicated no relevant financial relationships.
Parvez Mantry: Abbvie – Advisor or Review Panel Member, Grant/Research Support. Akero – Grant/Research Support. Aldeyra Sciences – Grant/Research Support. Amgen – Consultant, Speakers Bureau. BMS – Grant/Research Support, Speakers Bureau. Boston Scientific – Advisory Committee/Board Member, Speakers Bureau. Care Dx – Advisory Committee/Board Member, Grant/Research Support. Chemomab – Grant/Research Support. Cymabay – Advisory Committee/Board Member, Grant/Research Support. Eisai – Advisory Committee/Board Member, Speakers Bureau. Fibronostics – Grant/Research Support. Genentech – Grant/Research Support. Genfit – Grant/Research Support. Gilead – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Gore – Speakers Bureau. GSK – Grant/Research Support. Hanmi – Grant/Research Support. Hepquant – Advisor or Review Panel Member, Grant/Research Support. Intercept – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Inventiva – Grant/Research Support. Ipsen – Advisory Committee/Board Member, Speakers Bureau. Lipocine – Grant/Research Support. Madrigal – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Mallinckrodt – Advisory Committee/Board Member. Mirum – Grant/Research Support. Novo Nordisk – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Regneron – Grant/Research Support. Salix – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Sirtex – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Taiho – Grant/Research Support. Tempest Therapeutics – Grant/Research Support. Transthera Sciences – Grant/Research Support. Viking – Grant/Research Support.
Ashwini Mehta indicated no relevant financial relationships.
Judith Pozzerle indicated no relevant financial relationships.
Ali Khurram, MD1, Khadyoth Nanneboyina, MD1, Parvez Mantry, MD, CPE2, Ashwini Mehta, DO1, Judith Pozzerle, RN1. P3701 - The Impact of Systemic Chemoimmunotherapy in Combination With Locoregional Therapies on Transplant Rejection in Liver Transplant Secondary to Hepatocellular Carcinoma (HCC), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Ali Khurram, MD1, Khadyoth Nanneboyina, MD1, Parvez Mantry, MD, CPE2, Ashwini Mehta, DO1, Judith Pozzerle, RN1
1Dallas Methodist, Dallas, TX; 2Methodist Dallas Medical Center, Dallas, TX
Introduction: Liver transplantation (LT) is the gold standard treatment for unresectable HCC. However, many patients with HCC are outside of the Milan criteria for LT eligibility. In such cases, downstaging therapies decrease tumor burden to increase eligibility. Traditional downstaging methods include locoregional therapy (LRT) but recently, systemic therapy, particularly immune checkpoint inhibitors (ICIs), have shown promise. The use of ICIs in the treatment of HCC raises the concern for increased possibility of rejection following LT.
Methods: This study was a single-center retrospective outcome analysis of all patients diagnosed with HCC who underwent LT between June 2018 and March 2024 (n = 93) with primary endpoints being post-LT rejection and Rejection Activity Index (RAI) grade. Patients were categorized into 2 groups: 1) LRT alone and 2) combination of LRT and systemic therapy. LRT included Transarterial chemoembolization, Radioembolization, Microwave Ablation, and Stereotactic Beam Radiation Therapy. Systemic therapies included Nivolumab + Ipilimumab, Atezolizumab + Bevacizumab, Sorafenib, Lenvatinib, Ramuricumab, and Cabozantinib. T-test was performed.
Results: 78 patients received LRT alone and 15 patients received combination therapy. Median total tumor diameter on MRI prior to treatment initiation of the LRT group was 3 cm vs. 4.6 cm in the combination group (p = 0.02). Median alpha-fetoprotein (AFP) prior to treatment initiation of the LRT group was 5.4 ng/mL vs. 12.3 ng/mL in the combination group (p = 0.004). Within the combination group, 2 patients received Nivolumab + Ipilimumab, 7 received Atezolizumab + Bevacizumab, 6 received Sorafenib, 3 received Lenvatinib, 1 received Ramucirumab, and 2 received Cabozantinib. The incidence of transplant rejection in the LRT group was 22.6% vs. 20% in combination group (p = 0.43). The median RAI grade of rejection episodes in the LRT group was 5 vs. 6 in the combination group (p = 0.44). The incidence of serious infections requiring hospitalization in the LRT group was 15.4% vs. 6.7% in the combination group (p = 0.11).
Discussion: Our results show that combination therapy for the treatment of HCC was associated with similar rates of transplant rejection, RAI grade of rejection, and serious infections requiring hospitalization compared to patients who received LRT only. This preliminary data suggests that systemic therapies, such as ICIs, are safe to use for downstaging of HCC to potentially improve outcomes and increase transplant eligibility.

Figure: Comparison of LRT and combination patients
Disclosures:
Ali Khurram indicated no relevant financial relationships.
Khadyoth Nanneboyina indicated no relevant financial relationships.
Parvez Mantry: Abbvie – Advisor or Review Panel Member, Grant/Research Support. Akero – Grant/Research Support. Aldeyra Sciences – Grant/Research Support. Amgen – Consultant, Speakers Bureau. BMS – Grant/Research Support, Speakers Bureau. Boston Scientific – Advisory Committee/Board Member, Speakers Bureau. Care Dx – Advisory Committee/Board Member, Grant/Research Support. Chemomab – Grant/Research Support. Cymabay – Advisory Committee/Board Member, Grant/Research Support. Eisai – Advisory Committee/Board Member, Speakers Bureau. Fibronostics – Grant/Research Support. Genentech – Grant/Research Support. Genfit – Grant/Research Support. Gilead – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Gore – Speakers Bureau. GSK – Grant/Research Support. Hanmi – Grant/Research Support. Hepquant – Advisor or Review Panel Member, Grant/Research Support. Intercept – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Inventiva – Grant/Research Support. Ipsen – Advisory Committee/Board Member, Speakers Bureau. Lipocine – Grant/Research Support. Madrigal – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Mallinckrodt – Advisory Committee/Board Member. Mirum – Grant/Research Support. Novo Nordisk – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Regneron – Grant/Research Support. Salix – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Sirtex – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Taiho – Grant/Research Support. Tempest Therapeutics – Grant/Research Support. Transthera Sciences – Grant/Research Support. Viking – Grant/Research Support.
Ashwini Mehta indicated no relevant financial relationships.
Judith Pozzerle indicated no relevant financial relationships.
Ali Khurram, MD1, Khadyoth Nanneboyina, MD1, Parvez Mantry, MD, CPE2, Ashwini Mehta, DO1, Judith Pozzerle, RN1. P3701 - The Impact of Systemic Chemoimmunotherapy in Combination With Locoregional Therapies on Transplant Rejection in Liver Transplant Secondary to Hepatocellular Carcinoma (HCC), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

