Monday Poster Session
Category: Interventional Endoscopy
P3542 - Safety and Efficacy of Endoscopic Retrograde Cholangiopancreatography for Pancreatic Divisum in the Pediatric Population: Systematic Review and Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- TA
Tareq Alsaleh, MD
Department of Internal Medicine, AdventHealth Orlando
Orlando, FL
Presenting Author(s)
Tareq Alsaleh, MD1, Sagar J.. Pathak, MD2, Saurabh Chandan, MD2, Abdullah Abbasi, MD2, Shahab Khan, MBBS3, Ojasvini C.. Chandan, MD4, Maham Hayat, MD2, Deepanshu Jain, MD2, Natalie Cosgrove, MD2, Dennis Yang, MD, FACG5, Kambiz Kadkhodayan, MD2, Muhammad Hasan, MD, FACG2, Mustafa Arain, MD2
1Department of Internal Medicine, AdventHealth Orlando, Orlando, FL; 2Center for Interventional Endoscopy, AdventHealth Orlando, Orlando, FL; 3Brigham and Women's Hospital, Boston, MA; 4Department of Pediatric Gastroenterology, Texas Children's, Houston, TX; 5Center for Interventional Endoscopic, AdventHealth Orlando, Orlando, FL
Introduction: Pancreas divisum (PD) is the most common congenital anomaly of the pancreas. Although many PD patients are asymptomatic, some may experience recurrent acute pancreatitis (RAP), chronic pancreatitis (CP). Current evidence related to the utility of endoscopic retrograde cholangiopancreatography (ERCP) in PD has been mostly limited to adults. We conducted a systematic review and meta-analysis of the safety and efficacy of ERCP for PD in the pediatric population.
Methods: A systematic review of the literature from PubMed, Web of Science, Cochrane, EMBASE and Scopus was conducted from inception to February 2025, for studies assessing the safety and efficacy of ERCP in pediatric PD. The primary outcomes of interest was clinical success. Secondary outcomes included clinical remission, technical success, and post-ERCP pancreatitis. Pooled effect estimates were calculated using a random-effects model and expressed as proportions with 95% confidence intervals (CI). Heterogeneity was assessed using the I² statistic.
Results: A total of 8 retrospective studies were included that reported on 252 patients. Males comprised 39.7% of participants, and the median age ranged from 9 to 14 years. Median follow-up post-ERCP ranged from 12 to 41 months.
The pooled rate of clinical success was 83.7% (95% CI: 76.6%-90.8%; I2 = 42.53%). The pooled rate of clinical remission was 41.5% (95% CI: 18.6-64; I2 = 87.65%), while the pooled rate of technical success was 96.9% (95% CI: 94.3-99.6; I2 = 38.76%). The pooled rate of post-ERCP pancreatitis was 13.9% (95% CI: 7.9-20; I2 = 56.15%) (Figure 1).
In patients with PD presenting as recurrent acute pancreatitis (RAP), the pooled rate of clinical success was 81.6% (95% CI: 60.4-100; I2 = 91.3%), while in PD patients with chronic pancreatitis, the pooled rate of clinical success was 70.8% (95% CI: 58.3-83.3; I2 = 0%) (Figure 2).
Discussion: Our meta-analysis demonstrates that ERCP is an effective and safe procedure in pediatric patients with PD, particularly when presenting with RAP. It also has a high rate of technical success, but further large-scale prospective studies are needed to better understand its role and safety in this population.
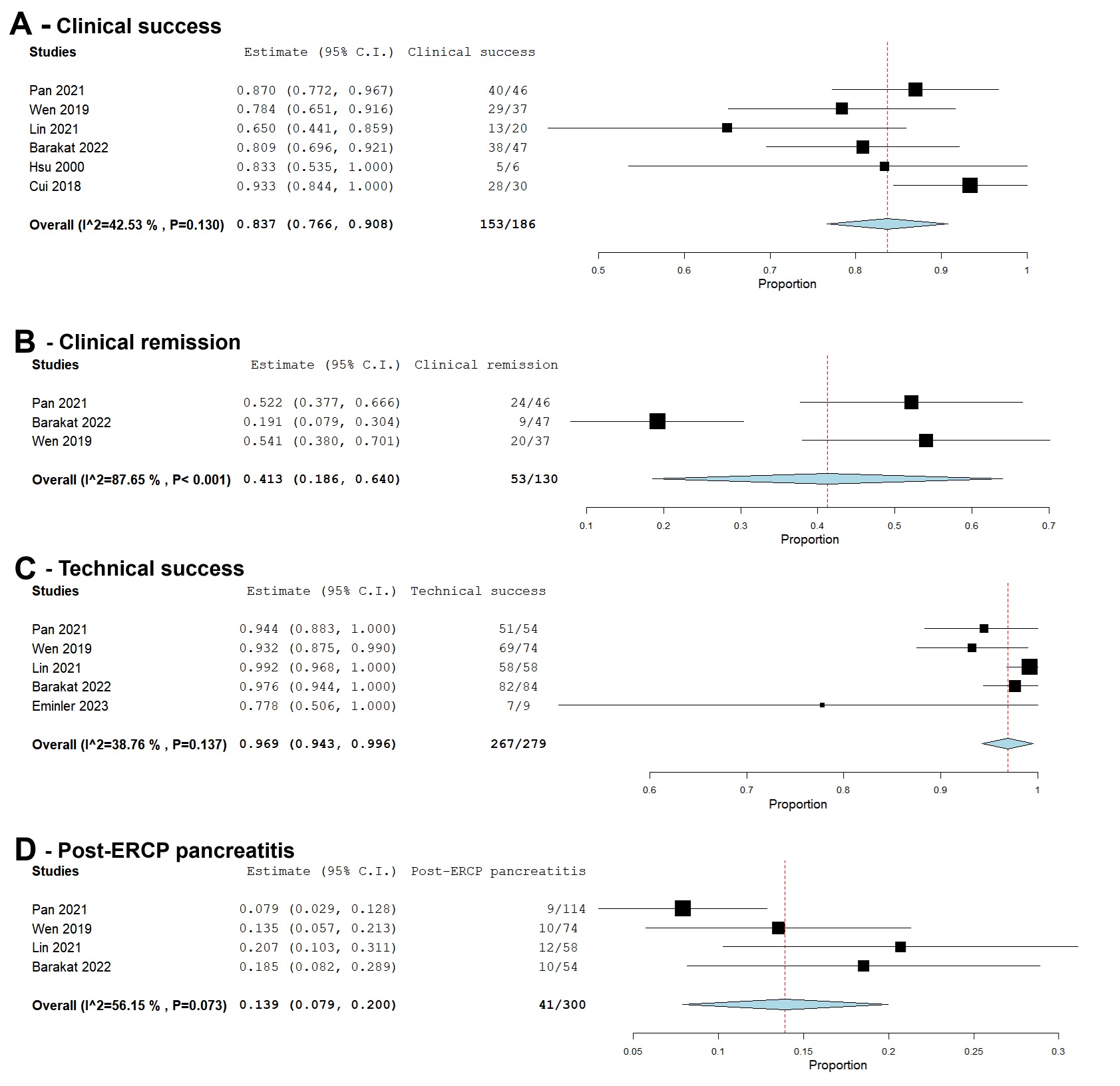
Figure: Figure 1. Forest plots showing pooled rates of:
A - Clinical success
B - Clinical remission
C - Technical success
D - Post-ERCP pancreatitis

Figure: Figure 1. Forest plots showing pooled rates of:
A - Clinical success in patients with recurrent acute pancreatitis
B - Clinical success in patients with chronic pancreatitis
Disclosures:
Tareq Alsaleh indicated no relevant financial relationships.
Sagar Pathak indicated no relevant financial relationships.
Saurabh Chandan indicated no relevant financial relationships.
Abdullah Abbasi indicated no relevant financial relationships.
Shahab Khan indicated no relevant financial relationships.
Ojasvini Chandan indicated no relevant financial relationships.
Maham Hayat indicated no relevant financial relationships.
Deepanshu Jain indicated no relevant financial relationships.
Natalie Cosgrove indicated no relevant financial relationships.
Dennis Yang: 3D-Matrix – Consultant. Apollo Endosurgery – Consultant. ERBE – Consultant. Fujifilm – Consultant. Medtronic – Consultant. MicroTech – Consultant. Olympus – Consultant.
Kambiz Kadkhodayan indicated no relevant financial relationships.
Muhammad Hasan: Boston Scientific – Consultant. MicroTech Endoscopy – Consultant. Olympus America – Consultant.
Mustafa Arain: Boston Scientific – Consultant. Cook Endoscopy – Consultant. Olympus – Consultant.
Tareq Alsaleh, MD1, Sagar J.. Pathak, MD2, Saurabh Chandan, MD2, Abdullah Abbasi, MD2, Shahab Khan, MBBS3, Ojasvini C.. Chandan, MD4, Maham Hayat, MD2, Deepanshu Jain, MD2, Natalie Cosgrove, MD2, Dennis Yang, MD, FACG5, Kambiz Kadkhodayan, MD2, Muhammad Hasan, MD, FACG2, Mustafa Arain, MD2. P3542 - Safety and Efficacy of Endoscopic Retrograde Cholangiopancreatography for Pancreatic Divisum in the Pediatric Population: Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Internal Medicine, AdventHealth Orlando, Orlando, FL; 2Center for Interventional Endoscopy, AdventHealth Orlando, Orlando, FL; 3Brigham and Women's Hospital, Boston, MA; 4Department of Pediatric Gastroenterology, Texas Children's, Houston, TX; 5Center for Interventional Endoscopic, AdventHealth Orlando, Orlando, FL
Introduction: Pancreas divisum (PD) is the most common congenital anomaly of the pancreas. Although many PD patients are asymptomatic, some may experience recurrent acute pancreatitis (RAP), chronic pancreatitis (CP). Current evidence related to the utility of endoscopic retrograde cholangiopancreatography (ERCP) in PD has been mostly limited to adults. We conducted a systematic review and meta-analysis of the safety and efficacy of ERCP for PD in the pediatric population.
Methods: A systematic review of the literature from PubMed, Web of Science, Cochrane, EMBASE and Scopus was conducted from inception to February 2025, for studies assessing the safety and efficacy of ERCP in pediatric PD. The primary outcomes of interest was clinical success. Secondary outcomes included clinical remission, technical success, and post-ERCP pancreatitis. Pooled effect estimates were calculated using a random-effects model and expressed as proportions with 95% confidence intervals (CI). Heterogeneity was assessed using the I² statistic.
Results: A total of 8 retrospective studies were included that reported on 252 patients. Males comprised 39.7% of participants, and the median age ranged from 9 to 14 years. Median follow-up post-ERCP ranged from 12 to 41 months.
The pooled rate of clinical success was 83.7% (95% CI: 76.6%-90.8%; I2 = 42.53%). The pooled rate of clinical remission was 41.5% (95% CI: 18.6-64; I2 = 87.65%), while the pooled rate of technical success was 96.9% (95% CI: 94.3-99.6; I2 = 38.76%). The pooled rate of post-ERCP pancreatitis was 13.9% (95% CI: 7.9-20; I2 = 56.15%) (Figure 1).
In patients with PD presenting as recurrent acute pancreatitis (RAP), the pooled rate of clinical success was 81.6% (95% CI: 60.4-100; I2 = 91.3%), while in PD patients with chronic pancreatitis, the pooled rate of clinical success was 70.8% (95% CI: 58.3-83.3; I2 = 0%) (Figure 2).
Discussion: Our meta-analysis demonstrates that ERCP is an effective and safe procedure in pediatric patients with PD, particularly when presenting with RAP. It also has a high rate of technical success, but further large-scale prospective studies are needed to better understand its role and safety in this population.

Figure: Figure 1. Forest plots showing pooled rates of:
A - Clinical success
B - Clinical remission
C - Technical success
D - Post-ERCP pancreatitis

Figure: Figure 1. Forest plots showing pooled rates of:
A - Clinical success in patients with recurrent acute pancreatitis
B - Clinical success in patients with chronic pancreatitis
Disclosures:
Tareq Alsaleh indicated no relevant financial relationships.
Sagar Pathak indicated no relevant financial relationships.
Saurabh Chandan indicated no relevant financial relationships.
Abdullah Abbasi indicated no relevant financial relationships.
Shahab Khan indicated no relevant financial relationships.
Ojasvini Chandan indicated no relevant financial relationships.
Maham Hayat indicated no relevant financial relationships.
Deepanshu Jain indicated no relevant financial relationships.
Natalie Cosgrove indicated no relevant financial relationships.
Dennis Yang: 3D-Matrix – Consultant. Apollo Endosurgery – Consultant. ERBE – Consultant. Fujifilm – Consultant. Medtronic – Consultant. MicroTech – Consultant. Olympus – Consultant.
Kambiz Kadkhodayan indicated no relevant financial relationships.
Muhammad Hasan: Boston Scientific – Consultant. MicroTech Endoscopy – Consultant. Olympus America – Consultant.
Mustafa Arain: Boston Scientific – Consultant. Cook Endoscopy – Consultant. Olympus – Consultant.
Tareq Alsaleh, MD1, Sagar J.. Pathak, MD2, Saurabh Chandan, MD2, Abdullah Abbasi, MD2, Shahab Khan, MBBS3, Ojasvini C.. Chandan, MD4, Maham Hayat, MD2, Deepanshu Jain, MD2, Natalie Cosgrove, MD2, Dennis Yang, MD, FACG5, Kambiz Kadkhodayan, MD2, Muhammad Hasan, MD, FACG2, Mustafa Arain, MD2. P3542 - Safety and Efficacy of Endoscopic Retrograde Cholangiopancreatography for Pancreatic Divisum in the Pediatric Population: Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

