Monday Poster Session
Category: Interventional Endoscopy
P3507 - Impact of Proton Pump Inhibitor Use on Post-Drainage Outcomes Following Axios Stent Placement for Pancreatic Fluid Collections: A Multi-Institutional Real-World Data Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Ashujot K. Dang, MD
University of California Riverside School of Medicine
Riverside, CA
Presenting Author(s)
Ashujot K. Dang, MD1, Vikash Kumar, MD2, Khyati Bidani, MD3, Aalam Sohal, MD2, Pir Shah, MD4, Indu Srinivasan, MD2, Wael Youssef, MD2, Dalbir Sandhu, MD2
1University of California Riverside School of Medicine, Riverside, CA; 2Creighton University School of Medicine, Phoenix, AZ; 3Saint Peter's University Hospital / Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ; 4Creighton University Medical Center, Phoenix, AZ
Introduction: Proton pump inhibitors (PPIs) are frequently continued after endoscopic drainage of pancreatic fluid collections (PFCs) using lumen-apposing metal stents (LAMS), often due to prior prescriptions or perceived benefits. However, current guidelines do not recommend routine PPI use in this context, and its impact on clinical outcomes remains unclear. Observational studies have raised concerns that PPI use may delay fistula closure and increase the risk of complications such as infection, bleeding, or the need for necrosectomy. This study evaluates the association between post-procedural PPI use and adverse outcomes including infection, gastrointestinal (GI) bleeding, and pancreatic necrosectomy.
Methods: We conducted a retrospective cohort study using the TriNetX US Collaborative Network, comprising 69 healthcare organizations. Adult patients (≥18 years) with PFCs (ICD-10: K86.2/K86.3) who underwent endoscopic transmural drainage with a LAMS (CPT: 43240) were included. Two cohorts were defined based on PPI use within one month post-procedure: Cohort 1 (PPI users, n=2,805) and Cohort 2 (non-users, n=3,000). Propensity score matching was performed (1:1), resulting in two balanced cohorts of 2,184 patients each. Outcomes were assessed over a one-year period using risk analysis and Kaplan-Meier survival estimates.
Results: Post-procedural PPI use was associated with significantly higher risks of adverse outcomes. Infections occurred in 23.6% of PPI users vs. 12.7% of non-users (Risk Ratio [RR] 1.85, p< 0.001). GI bleeding occurred in 14.1% vs. 5.7% (RR 2.46, p< 0.001), and the need for pancreatic necrosectomy was 3.8% vs. 1.4% (RR 2.77, p< 0.001). Kaplan-Meier analysis showed lower survival probabilities for each outcome in the PPI cohort. Corresponding hazard ratios (HR) were significantly elevated: infection (HR 2.01), GI bleeding (HR 2.63), and necrosectomy (HR 2.82), all with p< 0.001.
Discussion: PPI administration following Axios stent placement for PFC drainage is associated with increased risks of infection, GI bleeding, and the need for necrosectomy. These findings call for a critical evaluation of routine PPI use in this setting and suggest a need for prospective studies to guide post-drainage management.
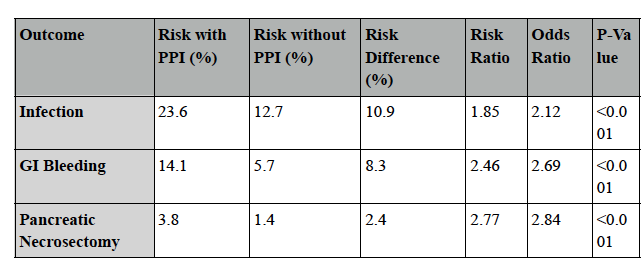
Figure: Table 1: Outcomes and adverse events among PPI vs non-PPI groups after axios stents
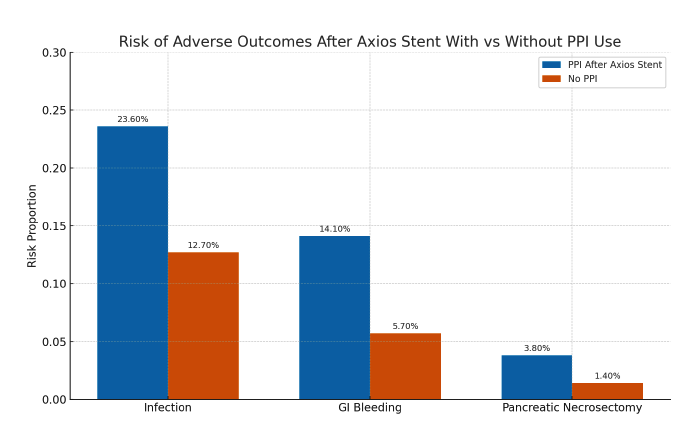
Figure: Figure 1: Risk of adverse outcomes after axios stent in PPI vs non-PPI cohorts
Disclosures:
Ashujot Dang indicated no relevant financial relationships.
Vikash Kumar indicated no relevant financial relationships.
Khyati Bidani indicated no relevant financial relationships.
Aalam Sohal indicated no relevant financial relationships.
Pir Shah indicated no relevant financial relationships.
Indu Srinivasan indicated no relevant financial relationships.
Wael Youssef indicated no relevant financial relationships.
Dalbir Sandhu indicated no relevant financial relationships.
Ashujot K. Dang, MD1, Vikash Kumar, MD2, Khyati Bidani, MD3, Aalam Sohal, MD2, Pir Shah, MD4, Indu Srinivasan, MD2, Wael Youssef, MD2, Dalbir Sandhu, MD2. P3507 - Impact of Proton Pump Inhibitor Use on Post-Drainage Outcomes Following Axios Stent Placement for Pancreatic Fluid Collections: A Multi-Institutional Real-World Data Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of California Riverside School of Medicine, Riverside, CA; 2Creighton University School of Medicine, Phoenix, AZ; 3Saint Peter's University Hospital / Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ; 4Creighton University Medical Center, Phoenix, AZ
Introduction: Proton pump inhibitors (PPIs) are frequently continued after endoscopic drainage of pancreatic fluid collections (PFCs) using lumen-apposing metal stents (LAMS), often due to prior prescriptions or perceived benefits. However, current guidelines do not recommend routine PPI use in this context, and its impact on clinical outcomes remains unclear. Observational studies have raised concerns that PPI use may delay fistula closure and increase the risk of complications such as infection, bleeding, or the need for necrosectomy. This study evaluates the association between post-procedural PPI use and adverse outcomes including infection, gastrointestinal (GI) bleeding, and pancreatic necrosectomy.
Methods: We conducted a retrospective cohort study using the TriNetX US Collaborative Network, comprising 69 healthcare organizations. Adult patients (≥18 years) with PFCs (ICD-10: K86.2/K86.3) who underwent endoscopic transmural drainage with a LAMS (CPT: 43240) were included. Two cohorts were defined based on PPI use within one month post-procedure: Cohort 1 (PPI users, n=2,805) and Cohort 2 (non-users, n=3,000). Propensity score matching was performed (1:1), resulting in two balanced cohorts of 2,184 patients each. Outcomes were assessed over a one-year period using risk analysis and Kaplan-Meier survival estimates.
Results: Post-procedural PPI use was associated with significantly higher risks of adverse outcomes. Infections occurred in 23.6% of PPI users vs. 12.7% of non-users (Risk Ratio [RR] 1.85, p< 0.001). GI bleeding occurred in 14.1% vs. 5.7% (RR 2.46, p< 0.001), and the need for pancreatic necrosectomy was 3.8% vs. 1.4% (RR 2.77, p< 0.001). Kaplan-Meier analysis showed lower survival probabilities for each outcome in the PPI cohort. Corresponding hazard ratios (HR) were significantly elevated: infection (HR 2.01), GI bleeding (HR 2.63), and necrosectomy (HR 2.82), all with p< 0.001.
Discussion: PPI administration following Axios stent placement for PFC drainage is associated with increased risks of infection, GI bleeding, and the need for necrosectomy. These findings call for a critical evaluation of routine PPI use in this setting and suggest a need for prospective studies to guide post-drainage management.

Figure: Table 1: Outcomes and adverse events among PPI vs non-PPI groups after axios stents

Figure: Figure 1: Risk of adverse outcomes after axios stent in PPI vs non-PPI cohorts
Disclosures:
Ashujot Dang indicated no relevant financial relationships.
Vikash Kumar indicated no relevant financial relationships.
Khyati Bidani indicated no relevant financial relationships.
Aalam Sohal indicated no relevant financial relationships.
Pir Shah indicated no relevant financial relationships.
Indu Srinivasan indicated no relevant financial relationships.
Wael Youssef indicated no relevant financial relationships.
Dalbir Sandhu indicated no relevant financial relationships.
Ashujot K. Dang, MD1, Vikash Kumar, MD2, Khyati Bidani, MD3, Aalam Sohal, MD2, Pir Shah, MD4, Indu Srinivasan, MD2, Wael Youssef, MD2, Dalbir Sandhu, MD2. P3507 - Impact of Proton Pump Inhibitor Use on Post-Drainage Outcomes Following Axios Stent Placement for Pancreatic Fluid Collections: A Multi-Institutional Real-World Data Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

