Monday Poster Session
Category: IBD
P3379 - Secukinumab-Induced Colitis Masquerading as Inflammatory Bowel Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Emelyn Martinez, MD (she/her/hers)
Hackensack Meridian Palisades Medical Center
North Bergen, NJ
Presenting Author(s)
Emelyn Martinez, MD1, Lahari Kota, MD2, Davood Karimi Hosseini, MD2, Chinmay Trivedi, MD2, Namrata Nikum, MD2
1Hackensack Meridian Palisades Medical Center, North Bergen, NJ; 2Hackensack University Medical Center, Hackensack, NJ
Introduction: Secukinumab is a human monoclonal antibody that targets interleukin-17A (IL-17A). While it is approved for rheumatologic skin diseases, its impact on gastrointestinal (GI) diseases remains controversial. In recent years, case reports have shown that use of secukinumab may lead to exacerbation of pre-existing Crohn's disease, unmasking of subclinical IBD, and de novo IBD-like colitis. Proposed mechanisms include disruption of mucosal barrier integrity and altered gut microbiome. We present a case of secukinumab-induced colitis in a patient without a prior history of IBD.
Case Description/
Methods: A 46-year-old female with a history of hidradenitis suppurativa (HS) presented with progressive abdominal pain, watery diarrhea with intermittent hematochezia, nausea, vomiting, and fatigue over one month. Notably, symptoms began approximately one month after initiating secukinumab for HS. Initial evaluation revealed leukocytosis with neutrophilic predominance, severe electrolyte depletion, and elevated inflammatory markers with a CRP of 18.6 and fecal lactoferrin of 150. Interestingly, fecal calprotectin was 40. CT abdomen-pelvis showed diffuse pancolitis with ascending colon predominance. C. difficile toxin and GI pathogen panel was negative. Colonoscopy performed on hospital day 5 demonstrated Mayo Grade 1 pancolitis; terminal ileum, R colon, L colon, and rectal biopsies were taken (Figure 1). Review of path from inpatient colonoscopy favored a diagnosis of Secukinumab induced colitis over IBD given a lack of chronicity, granulomas, or dysplasia, especially with acute inflammation, focal crypt abscesses, and crypt apoptotic bodies (Figure 2). A colonoscopy 6 months prior was unremarkable. Hence, a diagnosis of secukinumab-induced colitis was made. Secukinumab was discontinued and the patient’s symptoms subsequently improved. She was discharged in stable condition for outpatient follow-up.
Discussion: Secukinumab-induced colitis occurs in < 1% of those on treatment. However, it should be considered in the differential diagnosis for any patient presenting with new-onset GI symptoms while on therapy. Early recognition is critical and prompt discontinuation usually leads to symptom resolution. In severe or refractory cases, additional immunosuppressive therapy or corticosteroids may be required. This case underscores the importance of obtaining a detailed GI history prior to initiating secukinumab and maintaining a high index of suspicion for IBD-like complications during treatment with IL-17A inhibitors.
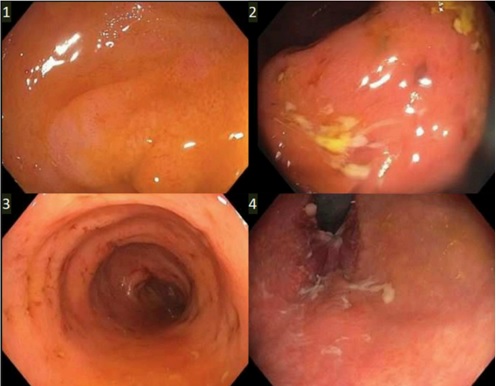
Figure: Figure 1: Colonoscopy Findings - Mayo Grade 1 pancolitis

Figure: Figure 2: Pathology report
Disclosures:
Emelyn Martinez indicated no relevant financial relationships.
Lahari Kota indicated no relevant financial relationships.
Davood Karimi Hosseini indicated no relevant financial relationships.
Chinmay Trivedi indicated no relevant financial relationships.
Namrata Nikum indicated no relevant financial relationships.
Emelyn Martinez, MD1, Lahari Kota, MD2, Davood Karimi Hosseini, MD2, Chinmay Trivedi, MD2, Namrata Nikum, MD2. P3379 - Secukinumab-Induced Colitis Masquerading as Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Hackensack Meridian Palisades Medical Center, North Bergen, NJ; 2Hackensack University Medical Center, Hackensack, NJ
Introduction: Secukinumab is a human monoclonal antibody that targets interleukin-17A (IL-17A). While it is approved for rheumatologic skin diseases, its impact on gastrointestinal (GI) diseases remains controversial. In recent years, case reports have shown that use of secukinumab may lead to exacerbation of pre-existing Crohn's disease, unmasking of subclinical IBD, and de novo IBD-like colitis. Proposed mechanisms include disruption of mucosal barrier integrity and altered gut microbiome. We present a case of secukinumab-induced colitis in a patient without a prior history of IBD.
Case Description/
Methods: A 46-year-old female with a history of hidradenitis suppurativa (HS) presented with progressive abdominal pain, watery diarrhea with intermittent hematochezia, nausea, vomiting, and fatigue over one month. Notably, symptoms began approximately one month after initiating secukinumab for HS. Initial evaluation revealed leukocytosis with neutrophilic predominance, severe electrolyte depletion, and elevated inflammatory markers with a CRP of 18.6 and fecal lactoferrin of 150. Interestingly, fecal calprotectin was 40. CT abdomen-pelvis showed diffuse pancolitis with ascending colon predominance. C. difficile toxin and GI pathogen panel was negative. Colonoscopy performed on hospital day 5 demonstrated Mayo Grade 1 pancolitis; terminal ileum, R colon, L colon, and rectal biopsies were taken (Figure 1). Review of path from inpatient colonoscopy favored a diagnosis of Secukinumab induced colitis over IBD given a lack of chronicity, granulomas, or dysplasia, especially with acute inflammation, focal crypt abscesses, and crypt apoptotic bodies (Figure 2). A colonoscopy 6 months prior was unremarkable. Hence, a diagnosis of secukinumab-induced colitis was made. Secukinumab was discontinued and the patient’s symptoms subsequently improved. She was discharged in stable condition for outpatient follow-up.
Discussion: Secukinumab-induced colitis occurs in < 1% of those on treatment. However, it should be considered in the differential diagnosis for any patient presenting with new-onset GI symptoms while on therapy. Early recognition is critical and prompt discontinuation usually leads to symptom resolution. In severe or refractory cases, additional immunosuppressive therapy or corticosteroids may be required. This case underscores the importance of obtaining a detailed GI history prior to initiating secukinumab and maintaining a high index of suspicion for IBD-like complications during treatment with IL-17A inhibitors.

Figure: Figure 1: Colonoscopy Findings - Mayo Grade 1 pancolitis

Figure: Figure 2: Pathology report
Disclosures:
Emelyn Martinez indicated no relevant financial relationships.
Lahari Kota indicated no relevant financial relationships.
Davood Karimi Hosseini indicated no relevant financial relationships.
Chinmay Trivedi indicated no relevant financial relationships.
Namrata Nikum indicated no relevant financial relationships.
Emelyn Martinez, MD1, Lahari Kota, MD2, Davood Karimi Hosseini, MD2, Chinmay Trivedi, MD2, Namrata Nikum, MD2. P3379 - Secukinumab-Induced Colitis Masquerading as Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
