Monday Poster Session
Category: IBD
P3345 - Upadacitinib vs Tofacitinib in the Management of Ulcerative Colitis: A Systematic Review and Meta-Analysis of Real-World Data
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- TA
Tareq Alsaleh, MD
Department of Internal Medicine, AdventHealth Orlando
Orlando, FL
Presenting Author(s)
Tareq Alsaleh, MD1, Aimen Farooq, MD2, Abdul Mohammed, MD2, Mohamad Khaled Almujarkesh, MD2, Mejia Lisandra, APN2, Donna Ortiz, DNP, APRN, FNP-C2, Babu P. Mohan, MD3, John George, MD2, Ilan Aharoni, MD2, Jennifer Seminerio, MD2
1Department of Internal Medicine, AdventHealth Orlando, Orlando, FL; 2Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL; 3Orlando Gastroenterology PA, Orlando, FL
Introduction: Upadacitinib and tofacitinib are Janus kinase (JAK) inhibitors that are safe and effective for the management of ulcerative colitis in biologic experienced individuals. Real-world data directly comparing the clinical outcomes of these agents is lacking. We conducted a systematic review and meta-analysis of the safety and efficacy of upadacitinib compared to tofacitinib in the management of ulcerative colitis.
Methods: A systematic review of the literature from PubMed, EMBASE, and Cochrane was performed through April 2025, for studies comparing upadacitinib and tofacitinib as induction or maintenance agents in ulcerative colitis. Outcomes of interest included corticosteroid-free clinical remission and adverse events. Standard meta-analysis methods were followed using the random-effects model. Treatment effect estimates were expressed as odds ratio (OR) and 95% confidence interval (CI). Heterogeneity was assessed using the I2% statistic.
Results: A total of 12 retrospective studies were included. Seven were journal articles, while five were conference abstracts. Propensity score matching was used in five studies. 889 patients received upadacitinib, while 1406 received tofacitinib. Males comprised 57.4% of participants. The odds of corticosteroid-free clinical remission (OR 2.83; 95% CI [2.14, 3.74], P< 0.001) and clinical response (OR 1.90; 95% CI [1.07, 3.39], P=0.03) were significantly higher in the upadacitinib group at the end of the study follow-up period. There was no statistically significant difference in the odds of biochemical remission (OR 1.18; 95% CI [0.39, 3.60], P=0.77) or endoscopic remission (OR 1.96; 95% CI [0.85, 4.51], P=0.12) at end of study follow-up (Figure 1).
The odds of treatment discontinuation were significantly lower with upadacitinib (OR 0.38; 95% CI [0.25, 0.58], P < 0.001). There was no statistically significant difference in the odds of adverse events between the two groups (OR 1.68; 95% CI [0.90, 3.14], P=0.10), but the odds of developing acne was significantly higher in the upadacitinib group (OR 7.55; 95% CI [2.13, 26.78], P=0.002) (Figure 2).
Discussion: Our meta-analysis demonstrates that upadacitinib is superior to tofacitinib in producing corticosteroid-free clinical remission and clinical response in ulcerative colitis, while having a similar safety profile and lower rates of treatment discontinuation. More high-quality prospective studies are needed to further compare the safety and efficacy outcomes of those two agents in this debilitating disease.
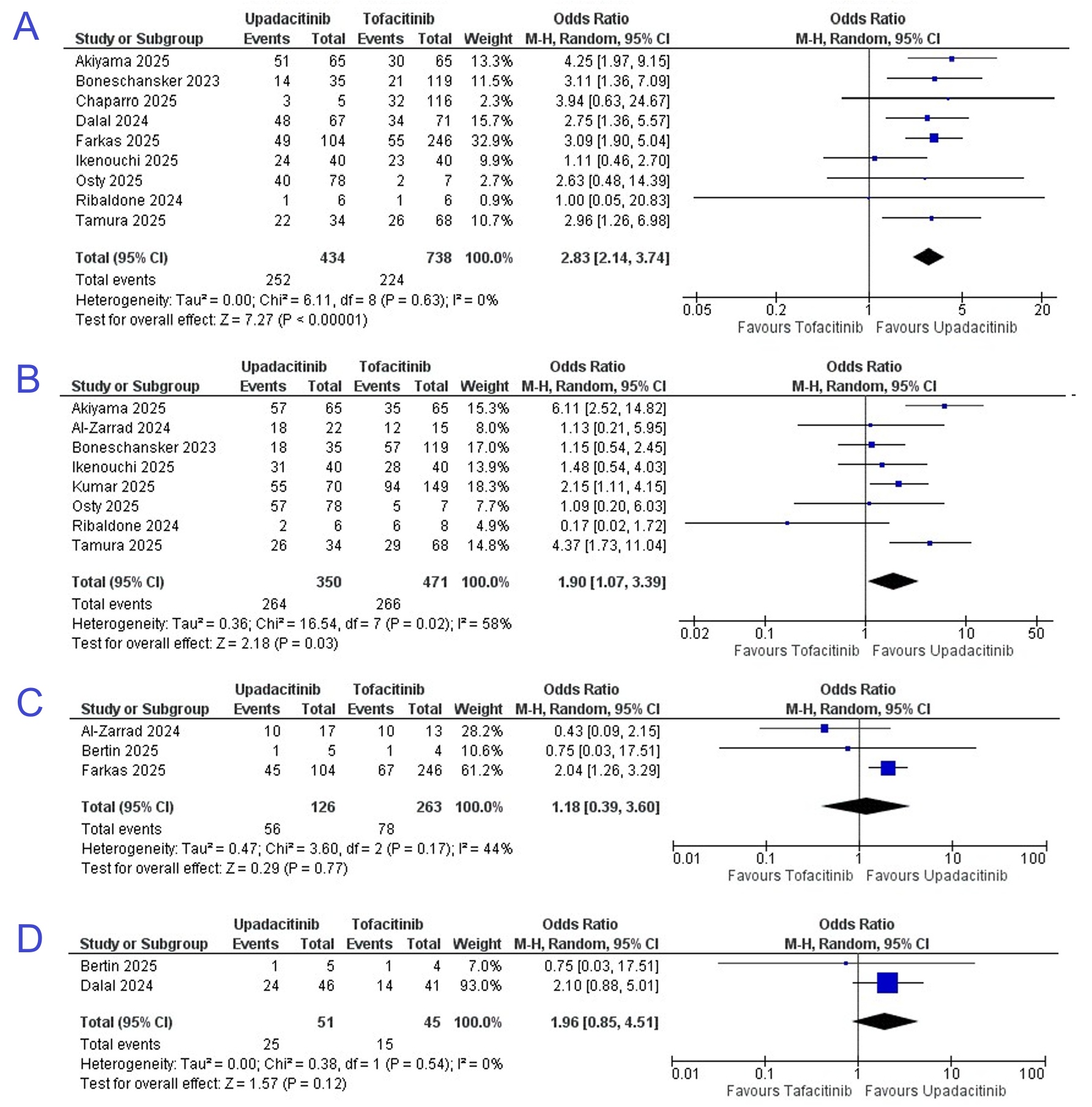
Figure: Figure 1. Pooled odds ratios (OR) between the upadacitinib and tofacitinib groups for:
A - Corticosteroid-free clinical remission
B - Clinical response
C - Biochemical remission
D - Endoscopic remission

Figure: Figure 2. Pooled odds ratios (OR) between the upadacitinib and tofacitinib groups for:
A - Treatment discontinuation
B - Overall adverse events
C - Acne
Disclosures:
Tareq Alsaleh indicated no relevant financial relationships.
Aimen Farooq indicated no relevant financial relationships.
Abdul Mohammed indicated no relevant financial relationships.
Mohamad Khaled Almujarkesh indicated no relevant financial relationships.
Mejia Lisandra indicated no relevant financial relationships.
Donna Ortiz: Abbvie – Consultant, Speakers Bureau. BMS – Speakers Bureau. Celltrion – Speakers Bureau. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Lilly – Consultant, Speakers Bureau.
Babu Mohan indicated no relevant financial relationships.
John George indicated no relevant financial relationships.
Ilan Aharoni indicated no relevant financial relationships.
Jennifer Seminerio: Abbvie – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant.
Tareq Alsaleh, MD1, Aimen Farooq, MD2, Abdul Mohammed, MD2, Mohamad Khaled Almujarkesh, MD2, Mejia Lisandra, APN2, Donna Ortiz, DNP, APRN, FNP-C2, Babu P. Mohan, MD3, John George, MD2, Ilan Aharoni, MD2, Jennifer Seminerio, MD2. P3345 - Upadacitinib vs Tofacitinib in the Management of Ulcerative Colitis: A Systematic Review and Meta-Analysis of Real-World Data, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Internal Medicine, AdventHealth Orlando, Orlando, FL; 2Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL; 3Orlando Gastroenterology PA, Orlando, FL
Introduction: Upadacitinib and tofacitinib are Janus kinase (JAK) inhibitors that are safe and effective for the management of ulcerative colitis in biologic experienced individuals. Real-world data directly comparing the clinical outcomes of these agents is lacking. We conducted a systematic review and meta-analysis of the safety and efficacy of upadacitinib compared to tofacitinib in the management of ulcerative colitis.
Methods: A systematic review of the literature from PubMed, EMBASE, and Cochrane was performed through April 2025, for studies comparing upadacitinib and tofacitinib as induction or maintenance agents in ulcerative colitis. Outcomes of interest included corticosteroid-free clinical remission and adverse events. Standard meta-analysis methods were followed using the random-effects model. Treatment effect estimates were expressed as odds ratio (OR) and 95% confidence interval (CI). Heterogeneity was assessed using the I2% statistic.
Results: A total of 12 retrospective studies were included. Seven were journal articles, while five were conference abstracts. Propensity score matching was used in five studies. 889 patients received upadacitinib, while 1406 received tofacitinib. Males comprised 57.4% of participants. The odds of corticosteroid-free clinical remission (OR 2.83; 95% CI [2.14, 3.74], P< 0.001) and clinical response (OR 1.90; 95% CI [1.07, 3.39], P=0.03) were significantly higher in the upadacitinib group at the end of the study follow-up period. There was no statistically significant difference in the odds of biochemical remission (OR 1.18; 95% CI [0.39, 3.60], P=0.77) or endoscopic remission (OR 1.96; 95% CI [0.85, 4.51], P=0.12) at end of study follow-up (Figure 1).
The odds of treatment discontinuation were significantly lower with upadacitinib (OR 0.38; 95% CI [0.25, 0.58], P < 0.001). There was no statistically significant difference in the odds of adverse events between the two groups (OR 1.68; 95% CI [0.90, 3.14], P=0.10), but the odds of developing acne was significantly higher in the upadacitinib group (OR 7.55; 95% CI [2.13, 26.78], P=0.002) (Figure 2).
Discussion: Our meta-analysis demonstrates that upadacitinib is superior to tofacitinib in producing corticosteroid-free clinical remission and clinical response in ulcerative colitis, while having a similar safety profile and lower rates of treatment discontinuation. More high-quality prospective studies are needed to further compare the safety and efficacy outcomes of those two agents in this debilitating disease.

Figure: Figure 1. Pooled odds ratios (OR) between the upadacitinib and tofacitinib groups for:
A - Corticosteroid-free clinical remission
B - Clinical response
C - Biochemical remission
D - Endoscopic remission

Figure: Figure 2. Pooled odds ratios (OR) between the upadacitinib and tofacitinib groups for:
A - Treatment discontinuation
B - Overall adverse events
C - Acne
Disclosures:
Tareq Alsaleh indicated no relevant financial relationships.
Aimen Farooq indicated no relevant financial relationships.
Abdul Mohammed indicated no relevant financial relationships.
Mohamad Khaled Almujarkesh indicated no relevant financial relationships.
Mejia Lisandra indicated no relevant financial relationships.
Donna Ortiz: Abbvie – Consultant, Speakers Bureau. BMS – Speakers Bureau. Celltrion – Speakers Bureau. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Lilly – Consultant, Speakers Bureau.
Babu Mohan indicated no relevant financial relationships.
John George indicated no relevant financial relationships.
Ilan Aharoni indicated no relevant financial relationships.
Jennifer Seminerio: Abbvie – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant.
Tareq Alsaleh, MD1, Aimen Farooq, MD2, Abdul Mohammed, MD2, Mohamad Khaled Almujarkesh, MD2, Mejia Lisandra, APN2, Donna Ortiz, DNP, APRN, FNP-C2, Babu P. Mohan, MD3, John George, MD2, Ilan Aharoni, MD2, Jennifer Seminerio, MD2. P3345 - Upadacitinib vs Tofacitinib in the Management of Ulcerative Colitis: A Systematic Review and Meta-Analysis of Real-World Data, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

