Monday Poster Session
Category: IBD
P3340 - Outcomes of Risankizumab in Patients with Crohn’s Disease and Prior Ustekinumab Exposure in a Multicenter Academic Institution
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Asrita Vattikonda, MD (she/her/hers)
Mayo Clinic
Jacksonville, FL
Presenting Author(s)
Asrita Vattikonda, MD, Tarek Odah, MD, MPH, Sheena Crosby, PharmD, Jana G. Hashash, MD, MSc, FACG, Jami Kinnucan, MD, FACG, Francis A.. Farraye, MD, MSc, MACG
Mayo Clinic, Jacksonville, FL
Introduction: Risankizumab (RZB) is a monoclonal antibody targeting interleukin (IL)-23 that is approved for the treatment of moderate to severe Crohn’s disease (CD). Many patients who start treatment with RZB were exposed to other biologics such as ustekinumab (UST), a previously approved monoclonal antibody that targets both IL-23 and IL-12. Our study aims to provide evidence on the safety and efficacy of RZB in patients exposed to UST.
Methods: This retrospective study evaluated adult patients (18 years or older) with CD who were prescribed RZB and had prior UST exposure at a multicenter academic institution between January 2022 and August 2024. Fisher’s exact test and Wilcoxon rank-sum test were used in the analysis.
Results: Among 68 patients treated with UST prior to RZB, 43 patients (63.2%) experienced symptomatic improvement on RZB, and 55 (80.9%) remained on RZB at the time of chart review for a mean duration of 12.7 (± 7.4) months. The mean time to documented improvement after starting RZB was 5.7 (± 5.1) months. Patients who did not improve on RZB were treated with a higher number of previous advanced CD medications (p = 0.011). There were no other differences in demographics and CD characteristics between patients who improved on RZB and those who did not (Table 1).
Among patients for whom UST was discontinued due to secondary non-response, 11 improved on RZB and 1 did not (p = 0.047). There was no difference in RZB response in patients who remained on the standard maintenance dose of UST (every 8 weeks) compared to those who underwent dose escalation to every 4 or 6 weeks prior to UST discontinuation (p = 0.557; Table 2).
Discussion: The results suggest that patients with CD and prior UST exposure who are treated with RZB can have favorable outcomes, though a higher number of previously trialed CD medications may predict a lack of improvement with RZB. The significantly higher RZB response among patients with secondary non-response to UST suggests that patients who experience loss of response to UST can still have positive outcomes on RZB. No difference in RZB outcomes was seen in patients who were on maintenance or escalated dosing of UST, suggesting that RZB is a reasonable treatment option in patients who do not respond to intensification of UST. Future prospective studies are essential to validate these findings and identify the subset of patients who would benefit from RZB after failure of UST.
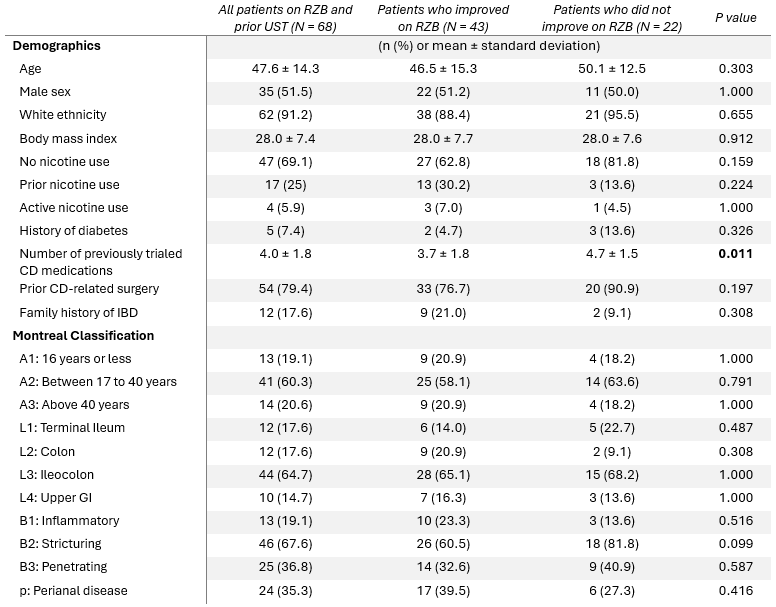
Figure: Table 1. Patient demographics and characteristics.
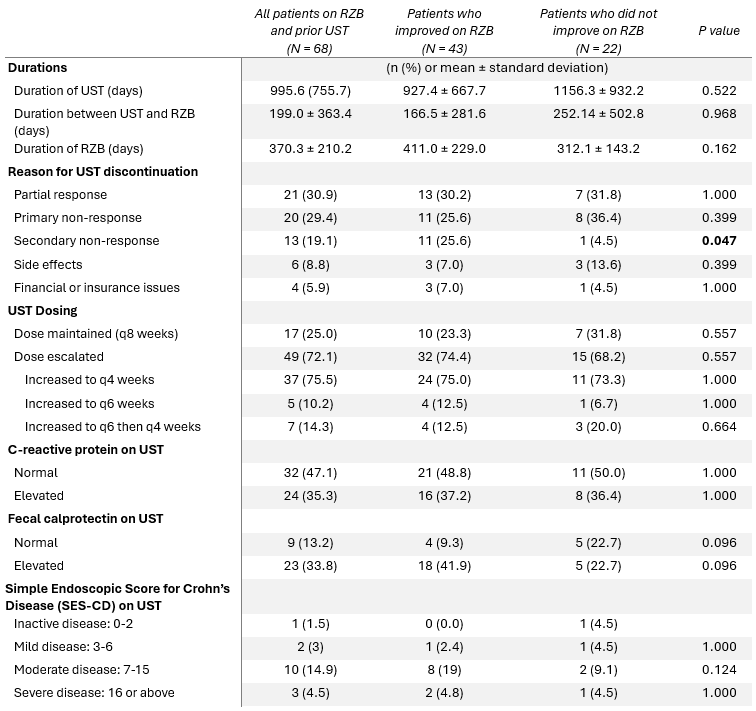
Figure: Table 2. UST characteristics prior to switching to RZB.
Disclosures:
Asrita Vattikonda indicated no relevant financial relationships.
Tarek Odah indicated no relevant financial relationships.
Sheena Crosby: Pharmacosmos – Advisory Committee/Board Member.
Jana Hashash: BMS – Ad Board.
Jami Kinnucan: Abbvie – Advisor or Review Panel Member, Consultant. J&J – Advisor or Review Panel Member. Lilly – Advisor or Review Panel Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Takeda – Advisor or Review Panel Member, Consultant.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Asrita Vattikonda, MD, Tarek Odah, MD, MPH, Sheena Crosby, PharmD, Jana G. Hashash, MD, MSc, FACG, Jami Kinnucan, MD, FACG, Francis A.. Farraye, MD, MSc, MACG. P3340 - Outcomes of Risankizumab in Patients with Crohn’s Disease and Prior Ustekinumab Exposure in a Multicenter Academic Institution, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Mayo Clinic, Jacksonville, FL
Introduction: Risankizumab (RZB) is a monoclonal antibody targeting interleukin (IL)-23 that is approved for the treatment of moderate to severe Crohn’s disease (CD). Many patients who start treatment with RZB were exposed to other biologics such as ustekinumab (UST), a previously approved monoclonal antibody that targets both IL-23 and IL-12. Our study aims to provide evidence on the safety and efficacy of RZB in patients exposed to UST.
Methods: This retrospective study evaluated adult patients (18 years or older) with CD who were prescribed RZB and had prior UST exposure at a multicenter academic institution between January 2022 and August 2024. Fisher’s exact test and Wilcoxon rank-sum test were used in the analysis.
Results: Among 68 patients treated with UST prior to RZB, 43 patients (63.2%) experienced symptomatic improvement on RZB, and 55 (80.9%) remained on RZB at the time of chart review for a mean duration of 12.7 (± 7.4) months. The mean time to documented improvement after starting RZB was 5.7 (± 5.1) months. Patients who did not improve on RZB were treated with a higher number of previous advanced CD medications (p = 0.011). There were no other differences in demographics and CD characteristics between patients who improved on RZB and those who did not (Table 1).
Among patients for whom UST was discontinued due to secondary non-response, 11 improved on RZB and 1 did not (p = 0.047). There was no difference in RZB response in patients who remained on the standard maintenance dose of UST (every 8 weeks) compared to those who underwent dose escalation to every 4 or 6 weeks prior to UST discontinuation (p = 0.557; Table 2).
Discussion: The results suggest that patients with CD and prior UST exposure who are treated with RZB can have favorable outcomes, though a higher number of previously trialed CD medications may predict a lack of improvement with RZB. The significantly higher RZB response among patients with secondary non-response to UST suggests that patients who experience loss of response to UST can still have positive outcomes on RZB. No difference in RZB outcomes was seen in patients who were on maintenance or escalated dosing of UST, suggesting that RZB is a reasonable treatment option in patients who do not respond to intensification of UST. Future prospective studies are essential to validate these findings and identify the subset of patients who would benefit from RZB after failure of UST.

Figure: Table 1. Patient demographics and characteristics.

Figure: Table 2. UST characteristics prior to switching to RZB.
Disclosures:
Asrita Vattikonda indicated no relevant financial relationships.
Tarek Odah indicated no relevant financial relationships.
Sheena Crosby: Pharmacosmos – Advisory Committee/Board Member.
Jana Hashash: BMS – Ad Board.
Jami Kinnucan: Abbvie – Advisor or Review Panel Member, Consultant. J&J – Advisor or Review Panel Member. Lilly – Advisor or Review Panel Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Takeda – Advisor or Review Panel Member, Consultant.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Asrita Vattikonda, MD, Tarek Odah, MD, MPH, Sheena Crosby, PharmD, Jana G. Hashash, MD, MSc, FACG, Jami Kinnucan, MD, FACG, Francis A.. Farraye, MD, MSc, MACG. P3340 - Outcomes of Risankizumab in Patients with Crohn’s Disease and Prior Ustekinumab Exposure in a Multicenter Academic Institution, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

