Monday Poster Session
Category: IBD
P3331 - An Update on a Multi-Center, Prospective, Non-Interventional Study of the Real-World Effectiveness of Etrasimod in Patients With Ulcerative Colitis (ENDEAVOUR-UC) Living in the United States
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Jordan Axelrad, MD, MPH
Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine
New York, NY
Presenting Author(s)
Jordan Axelrad, MD, MPH1, Hans Herfarth, MD, PhD2, Sharon Rimon, MSN, FNP3, Christina Cognata, PharmD, MBA4, Peter Hur, PharmD5, Elke Binder, PhD6, David Gruben, PhD7, Nicole Kulisek, MD8, Millie D. Long, MD, FACG9
1Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; 2University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 3Reddy GI Associates, Mesa, AZ, USA, Mesa, AZ; 4Pfizer Inc, Collegeville, PA; 5Pfizer Inc, New York, NY; 6Pfizer S.L.U., Alcobendas, Madrid, Spain, Madrid, Madrid, Spain; 7Pfizer Inc, Groton, CT; 8Pfizer Inc, Collegeville, PA, USA, Collegeville, PA; 9Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC
Introduction: Etrasimod is an oral, once-daily, selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). ENDEAVOUR-UC (NCT06398626) is a prospective, multi-center, non-interventional cohort study evaluating the real-world (RW) effectiveness of etrasimod in adults with moderately to severely active UC in the United States (US). High-quality observational studies remain important to understand the etrasimod RW experience. ENDEAVOUR-UC enrolled its first patient in September 2024. This is an update.
Methods: Eligible patients are aged ≥ 18 and < 65 years and begin treatment for moderately to severely active UC as part of routine clinical care, with any disease extent (Figure). Around 250–300 patients will be enrolled from 30 US sites. ELEVATE UC phase 3 randomized controlled trial data informed sample size, based on an assumed 40% Week 12 symptomatic remission rate for achieving a 6.1% precision level (half-width of 95% confidence interval). The study includes 52 weeks of follow-up from etrasimod initiation, with 28-day safety follow-up. Baseline patient and disease characteristics are collected. Patients are evaluated overall and by line of prior therapy exposure. Endpoint and patient-reported outcome (PRO) data are collected via a web-based application at baseline, daily for two weeks, then at Weeks 4, 8, 12, 24, 36, and 52. The primary endpoint is symptomatic remission at Week 12. Secondary endpoints include symptomatic remission, steroid-free symptomatic remission, and steroid-free clinical remission at Weeks 24, 36, and 52 using the partial modified Mayo score (stool frequency + rectal bleeding scores) and symptomatic response, clinical remission, changes in fatigue, bowel urgency, and abdominal pain at Weeks 12, 24, 36, and 52. Additional data (endoscopy, fecal calprotectin, complete blood count) are collected and analyzed if part of standard of care.
Results: As of April 2025, 30 sites have been selected, 23 are recruiting, and 25 patients are enrolled. Initial results on early-enrolled patients are expected in 2026, and final results in 2028. Principal investigators are diverse in geography and practice type to enhance patient diversity.
Discussion: ENDEAVOUR-UC will provide insights on early effectiveness and durability of etrasimod in a real-world setting, with PROs efficiently captured in real time via an innovative, specialized web-based application.
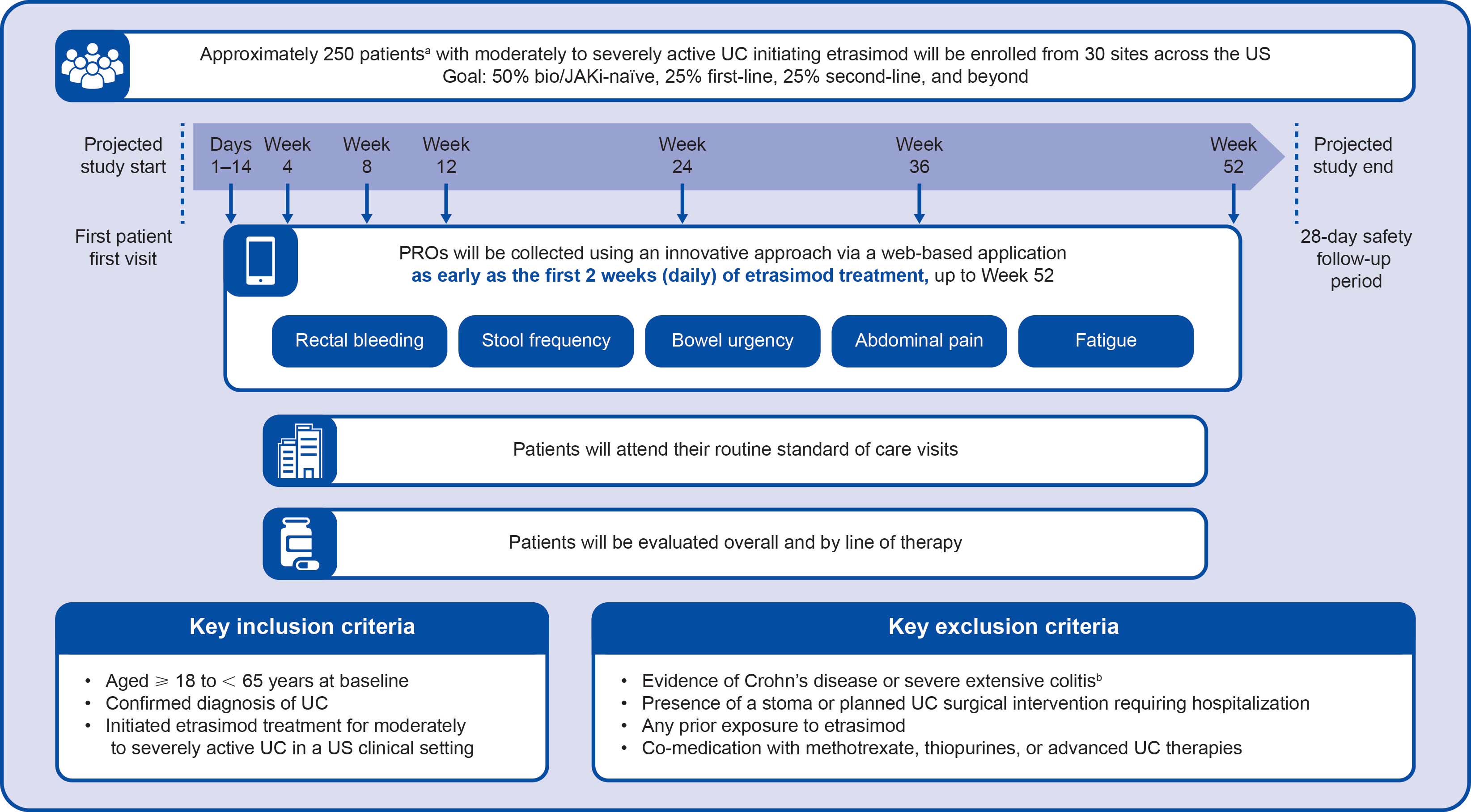
Figure: Figure. ENDEAVOUR-UC study design
[a]Sample size was informed by data from the ELEVATE UC phase 3 randomized controlled trials and assumes a symptomatic remission rate of 40% at Week 12 to achieve a 6.1% precision level (half-width of 95% confidence interval).
[b]Scored 0–3, with higher scores indicating more severe disease activity.
[c]Scored using an 11-point horizontal numerical rating scale, with higher scores indicating more severe bowel urgency.
[d]Scored using an 11-point horizontal numerical rating scale, with higher scores indicating more severe abdominal pain.
[e]Scored using the FACIT Fatigue questionnaire, with higher scores indicating less severe fatigue.
[f]Severe extensive colitis will be evidenced by physician judgment that the patient is likely to require hospitalization for medical or surgical intervention of any kind for UC (eg colectomy) within 12 weeks, or current evidence of acute severe UC, fulminant colitis, or toxic megacolon. bio/JAKi, biologic/Janus kinase inhibitor; FACIT, Functional Assessment of Chronic Illness Therapy; PRO, patient-reported outcome; UC, ulcerative colitis; US, United States.
Disclosures:
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Hans Herfarth: Celltrion – Consultant. ExeGi – Consultant. Gilead – Consultant. Janssen – Consultant. Lilly – Grant/Research Support. Novo Nordisk – Grant/Research Support.
Sharon Rimon: Pfizer Inc – Speakers Bureau.
Christina Cognata: Pfizer Inc – Employee, Stock Options.
Peter Hur: AbbVie – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Buhlmann – Grant/Research Support. Clene Nanomedicine – Stock Options. Haleon – Stock Options. Idorsia – Stock Options. Janssen – Grant/Research Support. Lilly – Grant/Research Support. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Grant/Research Support, Stock Options. Proctor & Gamble – Stock Options. Takeda – Grant/Research Support. US 2022/0257594 A1 – Intellectual Property/Patents.
Elke Binder: Pfizer Inc – Stock Options. Pfizer S.L.U. – Employee.
David Gruben: Pfizer Inc – Employee, Stock Options.
Nicole Kulisek: Pfizer Inc – Employee, Stock Options.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Jordan Axelrad, MD, MPH1, Hans Herfarth, MD, PhD2, Sharon Rimon, MSN, FNP3, Christina Cognata, PharmD, MBA4, Peter Hur, PharmD5, Elke Binder, PhD6, David Gruben, PhD7, Nicole Kulisek, MD8, Millie D. Long, MD, FACG9. P3331 - An Update on a Multi-Center, Prospective, Non-Interventional Study of the Real-World Effectiveness of Etrasimod in Patients With Ulcerative Colitis (ENDEAVOUR-UC) Living in the United States, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; 2University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 3Reddy GI Associates, Mesa, AZ, USA, Mesa, AZ; 4Pfizer Inc, Collegeville, PA; 5Pfizer Inc, New York, NY; 6Pfizer S.L.U., Alcobendas, Madrid, Spain, Madrid, Madrid, Spain; 7Pfizer Inc, Groton, CT; 8Pfizer Inc, Collegeville, PA, USA, Collegeville, PA; 9Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC
Introduction: Etrasimod is an oral, once-daily, selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). ENDEAVOUR-UC (NCT06398626) is a prospective, multi-center, non-interventional cohort study evaluating the real-world (RW) effectiveness of etrasimod in adults with moderately to severely active UC in the United States (US). High-quality observational studies remain important to understand the etrasimod RW experience. ENDEAVOUR-UC enrolled its first patient in September 2024. This is an update.
Methods: Eligible patients are aged ≥ 18 and < 65 years and begin treatment for moderately to severely active UC as part of routine clinical care, with any disease extent (Figure). Around 250–300 patients will be enrolled from 30 US sites. ELEVATE UC phase 3 randomized controlled trial data informed sample size, based on an assumed 40% Week 12 symptomatic remission rate for achieving a 6.1% precision level (half-width of 95% confidence interval). The study includes 52 weeks of follow-up from etrasimod initiation, with 28-day safety follow-up. Baseline patient and disease characteristics are collected. Patients are evaluated overall and by line of prior therapy exposure. Endpoint and patient-reported outcome (PRO) data are collected via a web-based application at baseline, daily for two weeks, then at Weeks 4, 8, 12, 24, 36, and 52. The primary endpoint is symptomatic remission at Week 12. Secondary endpoints include symptomatic remission, steroid-free symptomatic remission, and steroid-free clinical remission at Weeks 24, 36, and 52 using the partial modified Mayo score (stool frequency + rectal bleeding scores) and symptomatic response, clinical remission, changes in fatigue, bowel urgency, and abdominal pain at Weeks 12, 24, 36, and 52. Additional data (endoscopy, fecal calprotectin, complete blood count) are collected and analyzed if part of standard of care.
Results: As of April 2025, 30 sites have been selected, 23 are recruiting, and 25 patients are enrolled. Initial results on early-enrolled patients are expected in 2026, and final results in 2028. Principal investigators are diverse in geography and practice type to enhance patient diversity.
Discussion: ENDEAVOUR-UC will provide insights on early effectiveness and durability of etrasimod in a real-world setting, with PROs efficiently captured in real time via an innovative, specialized web-based application.

Figure: Figure. ENDEAVOUR-UC study design
[a]Sample size was informed by data from the ELEVATE UC phase 3 randomized controlled trials and assumes a symptomatic remission rate of 40% at Week 12 to achieve a 6.1% precision level (half-width of 95% confidence interval).
[b]Scored 0–3, with higher scores indicating more severe disease activity.
[c]Scored using an 11-point horizontal numerical rating scale, with higher scores indicating more severe bowel urgency.
[d]Scored using an 11-point horizontal numerical rating scale, with higher scores indicating more severe abdominal pain.
[e]Scored using the FACIT Fatigue questionnaire, with higher scores indicating less severe fatigue.
[f]Severe extensive colitis will be evidenced by physician judgment that the patient is likely to require hospitalization for medical or surgical intervention of any kind for UC (eg colectomy) within 12 weeks, or current evidence of acute severe UC, fulminant colitis, or toxic megacolon. bio/JAKi, biologic/Janus kinase inhibitor; FACIT, Functional Assessment of Chronic Illness Therapy; PRO, patient-reported outcome; UC, ulcerative colitis; US, United States.
Disclosures:
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Hans Herfarth: Celltrion – Consultant. ExeGi – Consultant. Gilead – Consultant. Janssen – Consultant. Lilly – Grant/Research Support. Novo Nordisk – Grant/Research Support.
Sharon Rimon: Pfizer Inc – Speakers Bureau.
Christina Cognata: Pfizer Inc – Employee, Stock Options.
Peter Hur: AbbVie – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Buhlmann – Grant/Research Support. Clene Nanomedicine – Stock Options. Haleon – Stock Options. Idorsia – Stock Options. Janssen – Grant/Research Support. Lilly – Grant/Research Support. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Grant/Research Support, Stock Options. Proctor & Gamble – Stock Options. Takeda – Grant/Research Support. US 2022/0257594 A1 – Intellectual Property/Patents.
Elke Binder: Pfizer Inc – Stock Options. Pfizer S.L.U. – Employee.
David Gruben: Pfizer Inc – Employee, Stock Options.
Nicole Kulisek: Pfizer Inc – Employee, Stock Options.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Jordan Axelrad, MD, MPH1, Hans Herfarth, MD, PhD2, Sharon Rimon, MSN, FNP3, Christina Cognata, PharmD, MBA4, Peter Hur, PharmD5, Elke Binder, PhD6, David Gruben, PhD7, Nicole Kulisek, MD8, Millie D. Long, MD, FACG9. P3331 - An Update on a Multi-Center, Prospective, Non-Interventional Study of the Real-World Effectiveness of Etrasimod in Patients With Ulcerative Colitis (ENDEAVOUR-UC) Living in the United States, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
