Monday Poster Session
Category: IBD
Impact of Immunosuppression on Recurrence of <i>Clostridioides difficile</i> Infection in Inflammatory Bowel Disease
P3328 - Impact of Immunosuppression on Recurrence of Clostridioides difficile Infection in Inflammatory Bowel Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Silpa Choday, MD (she/her/hers)
Creighton University School of Medicine
Phoenix, AZ
Presenting Author(s)
Silpa Choday, MD1, Keng-Yu Chuang, MD2, Laura Cicani, MD3, Gautam Maddineni, MD4, Akanksha Togra, MD5, Osama Abdur Rehman, MD4, Vikash Kumar, MD1, Wael Youssef, MD1, Dalbir Sandhu, MD1
1Creighton University School of Medicine, Phoenix, AZ; 2Arizona Digestive Health, GI Alliance, Glendale, AZ; 3International University of Health Sciences, Las Vegas, NV; 4Florida State University, Cape Coral, FL; 5Texas Tech University Health Sciences Center, El Paso, El Paso, TX
Introduction: Clostridioides difficile infection (CDI) is associated with poor outcomes in patients with inflammatory bowel diseases (IBD). Despite clinical awareness, real-world trends in immunosuppressive therapy use and outcomes among IBD patients with CDI remain underexplored. We conducted a nationally representative cohort study to evaluate the impact of recurrent CDI -related hospitalization and evaluated the trends in immunosuppressive treatment.
Methods: A retrospective analysis was conducted using national readmission data across six years (2016–2021). IBD patients were stratified by CDI status. Trends in steroid use, biologics, chemotherapy, transplant history, HIV status, mortality, LOS, and hospitalization charges were analyzed. Readmission rates at 30, 90, and 180 days were compared. P-values were calculated to assess statistical significance.
Results: We identified a total of 85,660 hospital readmissions involving patients with IBD and CDI. Of these, 26,704 occurred within 30 days, 41,855 within 90 days, and 49,032 within 180 days (P < 0.001). The average patient age was 55 years, with a female predominance (58%). Common comorbidities among readmitted patients included sepsis, acute kidney injury, and pneumonia.
Among IBD patients, those with CDI had significantly higher rates of biologic use (16% vs. 15%; P < 0.001) and steroid use (9.0% vs. 6.0%; P = 0.01) compared to those without CDI. CDI-IBD patients also showed increased readmission rates at 30 days (10.4%), 90 days (10.5%), and 180 days (10.4%) compared to non-CDI IBD patients (6.0%; P = 0.01).
Mortality was significantly higher in the CDI group (4.0% vs. 1.7%, P = 0.001), as were average length of stay (8.7 vs. 5.6 days, P = 0.01) and total hospital charges ($90,471 vs. $66,187, P = 0.001). Although the use of chemotherapy, immunotherapy, solid organ transplant status, and HIV was more common among CDI patients, each remained under 1% individually.
Discussion: Patients with IBD and CDI experienced notably higher 30-, 90-, and 180-day hospital readmission rates compared to those without CDI. These patients were also more likely on biologic and steroid therapy, indicating greater disease severity. Furthermore, the CDI group exhibited increased mortality, longer hospital stays, and higher healthcare costs. Although rare, comorbid conditions such as chemotherapy exposure, immunotherapy use, organ transplantation, and HIV were also more prevalent in the CDI cohort.
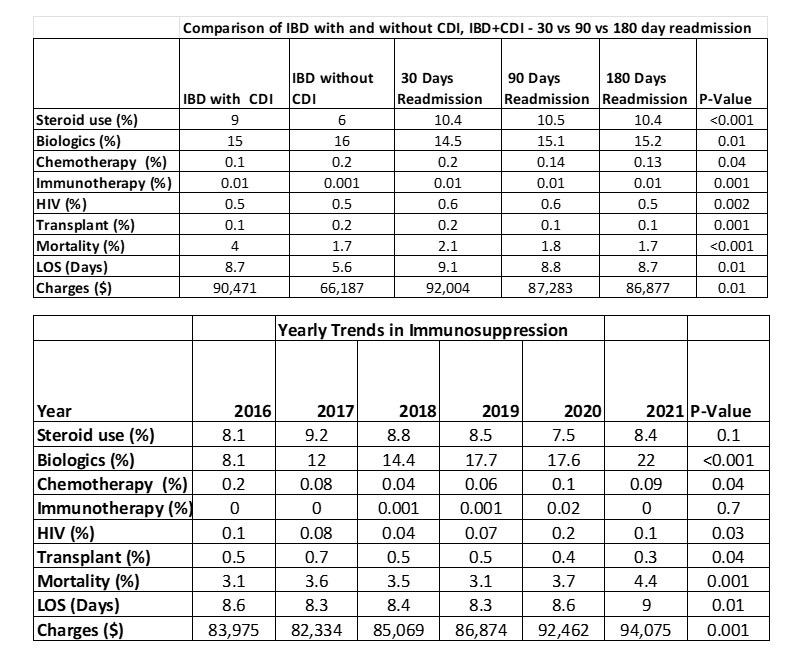
Figure: Table 1: Comparison of IBD with and without CDI, along with IBD+CDI - 30 vs 90 vs 180-day readmission and trends along the years
Figure: Figure1: IBD with CDI, 30 versus 90 versus 180 days readmission
Disclosures:
Silpa Choday indicated no relevant financial relationships.
Keng-Yu Chuang indicated no relevant financial relationships.
Laura Cicani indicated no relevant financial relationships.
Gautam Maddineni indicated no relevant financial relationships.
Akanksha Togra indicated no relevant financial relationships.
Osama Abdur Rehman indicated no relevant financial relationships.
Vikash Kumar indicated no relevant financial relationships.
Wael Youssef indicated no relevant financial relationships.
Dalbir Sandhu indicated no relevant financial relationships.
Silpa Choday, MD1, Keng-Yu Chuang, MD2, Laura Cicani, MD3, Gautam Maddineni, MD4, Akanksha Togra, MD5, Osama Abdur Rehman, MD4, Vikash Kumar, MD1, Wael Youssef, MD1, Dalbir Sandhu, MD1. P3328 - Impact of Immunosuppression on Recurrence of <i>Clostridioides difficile</i> Infection in Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Creighton University School of Medicine, Phoenix, AZ; 2Arizona Digestive Health, GI Alliance, Glendale, AZ; 3International University of Health Sciences, Las Vegas, NV; 4Florida State University, Cape Coral, FL; 5Texas Tech University Health Sciences Center, El Paso, El Paso, TX
Introduction: Clostridioides difficile infection (CDI) is associated with poor outcomes in patients with inflammatory bowel diseases (IBD). Despite clinical awareness, real-world trends in immunosuppressive therapy use and outcomes among IBD patients with CDI remain underexplored. We conducted a nationally representative cohort study to evaluate the impact of recurrent CDI -related hospitalization and evaluated the trends in immunosuppressive treatment.
Methods: A retrospective analysis was conducted using national readmission data across six years (2016–2021). IBD patients were stratified by CDI status. Trends in steroid use, biologics, chemotherapy, transplant history, HIV status, mortality, LOS, and hospitalization charges were analyzed. Readmission rates at 30, 90, and 180 days were compared. P-values were calculated to assess statistical significance.
Results: We identified a total of 85,660 hospital readmissions involving patients with IBD and CDI. Of these, 26,704 occurred within 30 days, 41,855 within 90 days, and 49,032 within 180 days (P < 0.001). The average patient age was 55 years, with a female predominance (58%). Common comorbidities among readmitted patients included sepsis, acute kidney injury, and pneumonia.
Among IBD patients, those with CDI had significantly higher rates of biologic use (16% vs. 15%; P < 0.001) and steroid use (9.0% vs. 6.0%; P = 0.01) compared to those without CDI. CDI-IBD patients also showed increased readmission rates at 30 days (10.4%), 90 days (10.5%), and 180 days (10.4%) compared to non-CDI IBD patients (6.0%; P = 0.01).
Mortality was significantly higher in the CDI group (4.0% vs. 1.7%, P = 0.001), as were average length of stay (8.7 vs. 5.6 days, P = 0.01) and total hospital charges ($90,471 vs. $66,187, P = 0.001). Although the use of chemotherapy, immunotherapy, solid organ transplant status, and HIV was more common among CDI patients, each remained under 1% individually.
Discussion: Patients with IBD and CDI experienced notably higher 30-, 90-, and 180-day hospital readmission rates compared to those without CDI. These patients were also more likely on biologic and steroid therapy, indicating greater disease severity. Furthermore, the CDI group exhibited increased mortality, longer hospital stays, and higher healthcare costs. Although rare, comorbid conditions such as chemotherapy exposure, immunotherapy use, organ transplantation, and HIV were also more prevalent in the CDI cohort.

Figure: Table 1: Comparison of IBD with and without CDI, along with IBD+CDI - 30 vs 90 vs 180-day readmission and trends along the years

Figure: Figure1: IBD with CDI, 30 versus 90 versus 180 days readmission
Disclosures:
Silpa Choday indicated no relevant financial relationships.
Keng-Yu Chuang indicated no relevant financial relationships.
Laura Cicani indicated no relevant financial relationships.
Gautam Maddineni indicated no relevant financial relationships.
Akanksha Togra indicated no relevant financial relationships.
Osama Abdur Rehman indicated no relevant financial relationships.
Vikash Kumar indicated no relevant financial relationships.
Wael Youssef indicated no relevant financial relationships.
Dalbir Sandhu indicated no relevant financial relationships.
Silpa Choday, MD1, Keng-Yu Chuang, MD2, Laura Cicani, MD3, Gautam Maddineni, MD4, Akanksha Togra, MD5, Osama Abdur Rehman, MD4, Vikash Kumar, MD1, Wael Youssef, MD1, Dalbir Sandhu, MD1. P3328 - Impact of Immunosuppression on Recurrence of <i>Clostridioides difficile</i> Infection in Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
