Monday Poster Session
Category: IBD
P3321 - Mirikizumab Improves Quality of Life in Patients With Moderately-to-Severely Active Ulcerative Colitis: Results From an Open Label Phase 3b Study (LUCENT-URGE)
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Millie D. Long, MD, FACG
Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Chapel Hill, NC
Presenting Author(s)
Millie D. Long, MD, FACG1, David Laharie, 2, Gerhard Rogler, MD, PhD3, Seyedehsan Navabi, MD4, William J. Eastman, 5, Sarah Folian, 5, Tian Tian, 5, Alison Potts Bleakman, 5, Alessandro Armuzzi, MD, PhD6
1Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 2CHU de Bordeaux, Centre Medico-chirurgical Magellan, Hôpital Haut-Lévêque, Gastroenterology Department; Université de Bordeaux, Pessac, Aquitaine, France; 3University Hospital Zurich, University of Zurich, Zurich, Zurich, Switzerland; 4Gastroenterology, United Medical Doctors, Murrieta, CA; 5Eli Lilly and Company, Indianapolis, IN; 6Humanitas University, Pieve Emanuele, Lombardia, Italy
Introduction: Mirikizumab (Miri), an anti-IL-23p19 monoclonal antibody, is approved for the treatment of ulcerative colitis (UC) and Crohn’s disease. LUCENT-URGE (NCT05767021), a phase 3b, open-label, single-arm study, evaluated the impact of Miri on bowel urgency (BU) through week (W) 28 in adults with moderately-to-severely active UC, using both novel measures of BU and validated instruments designed to assess quality of life (QoL).
Methods: Patients with UC who had baseline (BL) BU severity ≥3 on the Urgency Numeric Rating Scale (UNRS) were included. Participants received 300 mg Miri intravenously at W0, W4, and W8, followed by 200 mg subcutaneously at W12 and every 4W up to W28. Inflammatory Bowel Disease Questionnaire (IBDQ), Work Productivity and Activity Impairment (WPAI-UC), Wexner Incontinence Score (WIS), Sexual Activity Avoidance (SAA) due to UC symptoms, and Absorbent Product Use (APU) for BU were assessed at BL, W12, and W28. Discontinuations or missing data were addressed by non-responder imputation and BL observation carried forward. Descriptive statistics were used for continuous measures and proportions for binary outcomes. A ≥3-point reduction in UNRS score indicates a clinically meaningful improvement (CMI) in BU severity. IBDQ meaningful response and remission are ≥16-point increase and IBDQ ≥170, respectively.
Results: 172 patients with UC were enrolled in the study (Table). The mean UNRS score improved by 3.2 at W12 and by 3.6 at W28. CMI in BU severity was achieved by 53.5% of patients at W12 and by 58.1% at W28. The IBDQ score improved by 51.1 at W12 and by 57.1 at W28. IBDQ remission was achieved by 47.1% of patients at W12 and 52.3% at W28. Mean WPAI-UC activity impairment score improved by 29.3% at W12 and by 32.0% at W28. Mean WIS score improved by 3.8 at W12 and by 4.9 at W28. At BL, 55.1% of patients reported APU for BU, decreasing to 34.7% at W12 and 29.6% at W28, and those reporting no APU for BU increased from 44.9% at BL to 70.4% at W28. Proportion of patients reporting no SAA due to UC symptoms increased from 21.1% at BL to 65.0% at W28.
Discussion: Miri treatment led to clinically meaningful improvements in BU over 28 weeks. This improvement in BU severity was associated with meaningful improvement or remission over time in multiple validated QoL measures, and reduction or absence of negative behavioural impacts of BU. These findings suggest that targeting BU with Miri is an effective strategy for managing this burdensome symptom and patient-reported QoL measures.
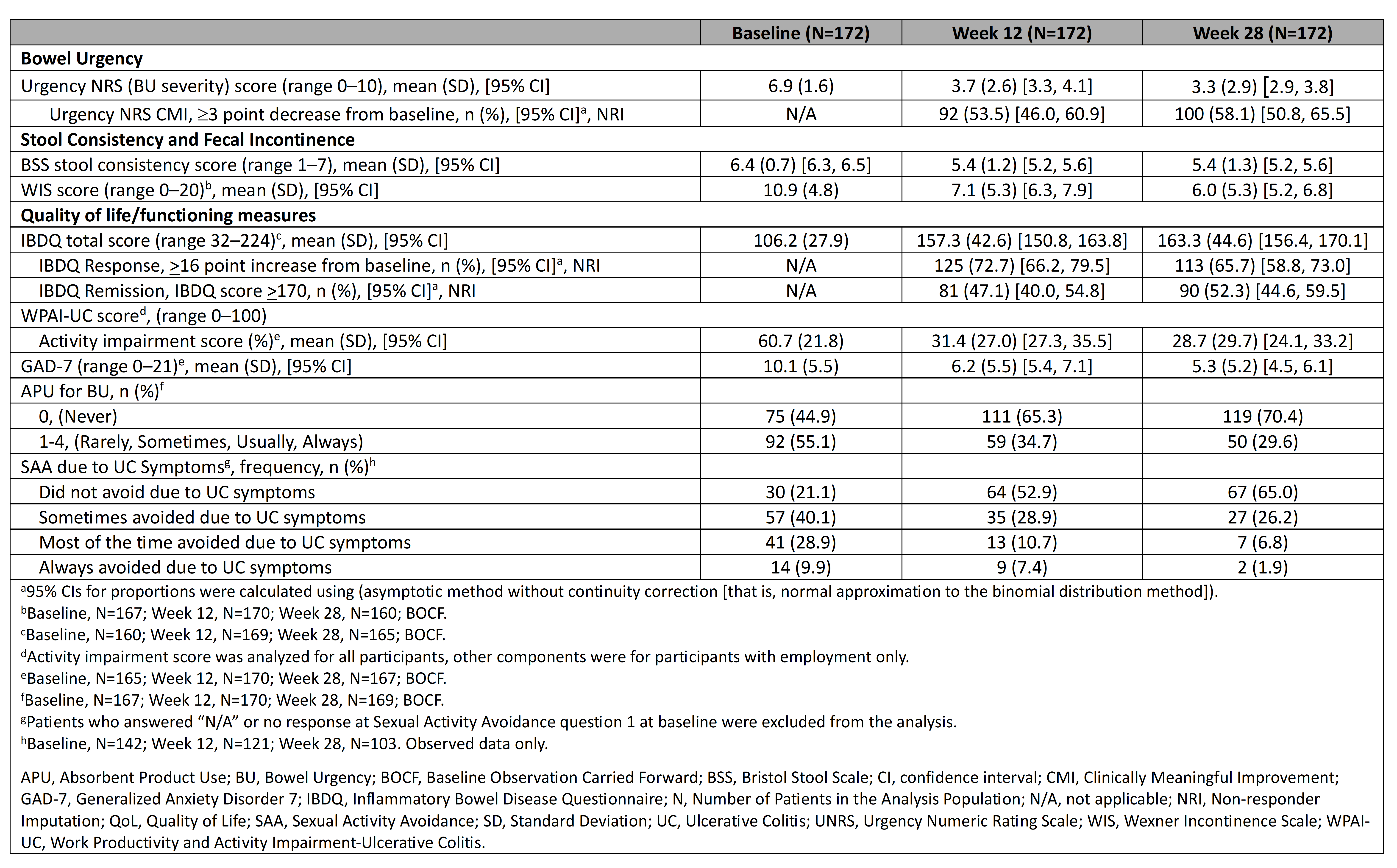
Figure: Table. Bowel urgency severity, UC symptoms, and QoL/Functioning at baseline, week 12, and week 28, BOCF
Disclosures:
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
David Laharie: AbbVie – Advisory Committee/Board Member, Consultant, Transport, Fees.. Alfasigma – Advisory Committee/Board Member, Consultant, Transport or Fees. Amgen – Advisory Committee/Board Member, Consultant, Transport, Fees.. Celltrion – Advisory Committee/Board Member, Consultant, Transport, Fees.. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Transport, Fees.. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Transport, Fees.. Janssen – Advisory Committee/Board Member, Consultant, Transport, Fees.. Medac – Advisory Committee/Board Member, Consultant, Transport or Fees. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Transport, Fees.. Pfizer – Advisory Committee/Board Member, Consultant, Transport, Fees.. Prometheus – Advisory Committee/Board Member, Consultant, Transport, Fees.. Takeda – Advisory Committee/Board Member, Consultant, Transport, Fees.. Theradiag – Advisory Committee/Board Member, Consultant, Transport, Fees..
Gerhard Rogler: Abbvie – Consultant, Grant/Research Support, Speakers Bureau. Ardeypharm – Grant/Research Support. Arrena – Consultant. Astra Zeneca – Speakers Bureau. Augurix – Consultant, Grant/Research Support. BMS – Consultant, Speakers Bureau. Boehringer – Consultant. Calypso – Consultant, Grant/Research Support. Celgene – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support. FALK – Consultant, Grant/Research Support, Speakers Bureau. Ferring – Consultant. Fisher – Consultant. Flamentera – Grant/Research Support. Genentech – Consultant. Gilead – Consultant. Janssen – Consultant, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Novartis – Consultant, Grant/Research Support. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Phadia – Consultant, Speakers Bureau. PharmaBiome – Cofounder and head of the scientific advisory board. Roche – Consultant, Grant/Research Support. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Tillots – Consultant, Grant/Research Support, Speakers Bureau. UCB – Consultant, Grant/Research Support, Speakers Bureau. Vifor – Consultant, Speakers Bureau. Vital Solutions – Consultant. Zeller – Consultant, Grant/Research Support, Speakers Bureau.
Seyedehsan Navabi: Eli Lilly and Company – Advisory Committee/Board Member, Speakers Bureau. Janssen – Speakers Bureau. Madrigal – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau.
William Eastman: Eli Lilly and Company – Employee, Stock Options.
Sarah Folian: Eli Lilly and Company – Employee, Stock Options.
Tian Tian: Eli Lilly and Company – Employee, Stock Options.
Alison Potts Bleakman: Eli Lilly and Company – Employee, Stock Options.
Alessandro Armuzzi: AbbVie – Consultant, Speakers Bureau. Allergan – Consultant. Amgen – Consultant, Speakers Bureau. Arena – Consultant, Speakers Bureau. Biogen – Consultant, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol-Myers Squibb – Consultant, Speakers Bureau. Celgene – Consultant. Celltrion – Consultant. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant. Novartis – Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant. Roche – Consultant, Speakers Bureau. Samsung Bioepis – Consultant, Speakers Bureau. Sandoz – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Tigenix – Speakers Bureau.
Millie D. Long, MD, FACG1, David Laharie, 2, Gerhard Rogler, MD, PhD3, Seyedehsan Navabi, MD4, William J. Eastman, 5, Sarah Folian, 5, Tian Tian, 5, Alison Potts Bleakman, 5, Alessandro Armuzzi, MD, PhD6. P3321 - Mirikizumab Improves Quality of Life in Patients With Moderately-to-Severely Active Ulcerative Colitis: Results From an Open Label Phase 3b Study (LUCENT-URGE), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 2CHU de Bordeaux, Centre Medico-chirurgical Magellan, Hôpital Haut-Lévêque, Gastroenterology Department; Université de Bordeaux, Pessac, Aquitaine, France; 3University Hospital Zurich, University of Zurich, Zurich, Zurich, Switzerland; 4Gastroenterology, United Medical Doctors, Murrieta, CA; 5Eli Lilly and Company, Indianapolis, IN; 6Humanitas University, Pieve Emanuele, Lombardia, Italy
Introduction: Mirikizumab (Miri), an anti-IL-23p19 monoclonal antibody, is approved for the treatment of ulcerative colitis (UC) and Crohn’s disease. LUCENT-URGE (NCT05767021), a phase 3b, open-label, single-arm study, evaluated the impact of Miri on bowel urgency (BU) through week (W) 28 in adults with moderately-to-severely active UC, using both novel measures of BU and validated instruments designed to assess quality of life (QoL).
Methods: Patients with UC who had baseline (BL) BU severity ≥3 on the Urgency Numeric Rating Scale (UNRS) were included. Participants received 300 mg Miri intravenously at W0, W4, and W8, followed by 200 mg subcutaneously at W12 and every 4W up to W28. Inflammatory Bowel Disease Questionnaire (IBDQ), Work Productivity and Activity Impairment (WPAI-UC), Wexner Incontinence Score (WIS), Sexual Activity Avoidance (SAA) due to UC symptoms, and Absorbent Product Use (APU) for BU were assessed at BL, W12, and W28. Discontinuations or missing data were addressed by non-responder imputation and BL observation carried forward. Descriptive statistics were used for continuous measures and proportions for binary outcomes. A ≥3-point reduction in UNRS score indicates a clinically meaningful improvement (CMI) in BU severity. IBDQ meaningful response and remission are ≥16-point increase and IBDQ ≥170, respectively.
Results: 172 patients with UC were enrolled in the study (Table). The mean UNRS score improved by 3.2 at W12 and by 3.6 at W28. CMI in BU severity was achieved by 53.5% of patients at W12 and by 58.1% at W28. The IBDQ score improved by 51.1 at W12 and by 57.1 at W28. IBDQ remission was achieved by 47.1% of patients at W12 and 52.3% at W28. Mean WPAI-UC activity impairment score improved by 29.3% at W12 and by 32.0% at W28. Mean WIS score improved by 3.8 at W12 and by 4.9 at W28. At BL, 55.1% of patients reported APU for BU, decreasing to 34.7% at W12 and 29.6% at W28, and those reporting no APU for BU increased from 44.9% at BL to 70.4% at W28. Proportion of patients reporting no SAA due to UC symptoms increased from 21.1% at BL to 65.0% at W28.
Discussion: Miri treatment led to clinically meaningful improvements in BU over 28 weeks. This improvement in BU severity was associated with meaningful improvement or remission over time in multiple validated QoL measures, and reduction or absence of negative behavioural impacts of BU. These findings suggest that targeting BU with Miri is an effective strategy for managing this burdensome symptom and patient-reported QoL measures.

Figure: Table. Bowel urgency severity, UC symptoms, and QoL/Functioning at baseline, week 12, and week 28, BOCF
Disclosures:
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
David Laharie: AbbVie – Advisory Committee/Board Member, Consultant, Transport, Fees.. Alfasigma – Advisory Committee/Board Member, Consultant, Transport or Fees. Amgen – Advisory Committee/Board Member, Consultant, Transport, Fees.. Celltrion – Advisory Committee/Board Member, Consultant, Transport, Fees.. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Transport, Fees.. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Transport, Fees.. Janssen – Advisory Committee/Board Member, Consultant, Transport, Fees.. Medac – Advisory Committee/Board Member, Consultant, Transport or Fees. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Transport, Fees.. Pfizer – Advisory Committee/Board Member, Consultant, Transport, Fees.. Prometheus – Advisory Committee/Board Member, Consultant, Transport, Fees.. Takeda – Advisory Committee/Board Member, Consultant, Transport, Fees.. Theradiag – Advisory Committee/Board Member, Consultant, Transport, Fees..
Gerhard Rogler: Abbvie – Consultant, Grant/Research Support, Speakers Bureau. Ardeypharm – Grant/Research Support. Arrena – Consultant. Astra Zeneca – Speakers Bureau. Augurix – Consultant, Grant/Research Support. BMS – Consultant, Speakers Bureau. Boehringer – Consultant. Calypso – Consultant, Grant/Research Support. Celgene – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support. FALK – Consultant, Grant/Research Support, Speakers Bureau. Ferring – Consultant. Fisher – Consultant. Flamentera – Grant/Research Support. Genentech – Consultant. Gilead – Consultant. Janssen – Consultant, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Novartis – Consultant, Grant/Research Support. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Phadia – Consultant, Speakers Bureau. PharmaBiome – Cofounder and head of the scientific advisory board. Roche – Consultant, Grant/Research Support. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Tillots – Consultant, Grant/Research Support, Speakers Bureau. UCB – Consultant, Grant/Research Support, Speakers Bureau. Vifor – Consultant, Speakers Bureau. Vital Solutions – Consultant. Zeller – Consultant, Grant/Research Support, Speakers Bureau.
Seyedehsan Navabi: Eli Lilly and Company – Advisory Committee/Board Member, Speakers Bureau. Janssen – Speakers Bureau. Madrigal – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau.
William Eastman: Eli Lilly and Company – Employee, Stock Options.
Sarah Folian: Eli Lilly and Company – Employee, Stock Options.
Tian Tian: Eli Lilly and Company – Employee, Stock Options.
Alison Potts Bleakman: Eli Lilly and Company – Employee, Stock Options.
Alessandro Armuzzi: AbbVie – Consultant, Speakers Bureau. Allergan – Consultant. Amgen – Consultant, Speakers Bureau. Arena – Consultant, Speakers Bureau. Biogen – Consultant, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol-Myers Squibb – Consultant, Speakers Bureau. Celgene – Consultant. Celltrion – Consultant. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant. Novartis – Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant. Roche – Consultant, Speakers Bureau. Samsung Bioepis – Consultant, Speakers Bureau. Sandoz – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Tigenix – Speakers Bureau.
Millie D. Long, MD, FACG1, David Laharie, 2, Gerhard Rogler, MD, PhD3, Seyedehsan Navabi, MD4, William J. Eastman, 5, Sarah Folian, 5, Tian Tian, 5, Alison Potts Bleakman, 5, Alessandro Armuzzi, MD, PhD6. P3321 - Mirikizumab Improves Quality of Life in Patients With Moderately-to-Severely Active Ulcerative Colitis: Results From an Open Label Phase 3b Study (LUCENT-URGE), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
