Monday Poster Session
Category: IBD
P3311 - Efficacy and Safety of Stem Cell Therapy in Treating Inflammatory Bowel Disease: A Comprehensive Meta-Analysis of Clinical Outcomes and Long-Term Effects
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- SS
Sohini Samaddar, MBBS (she/her/hers)
University of Kansas Medical Center
Kansas City, KS
Presenting Author(s)
Angad Tiwari, MBBS1, Sohini Samaddar, MBBS2, Harendra Kumar, MBBS3, Charlie Altfillisch, MD2, Dushyant Dahiya, MD2, Eric Molloy, MD4
1Maharani Laxmi Bai Medical College, Ghaziabad, Uttar Pradesh, India; 2University of Kansas Medical Center, Kansas City, KS; 3Dow University of Health Sciences, Karachi, Sindh, Pakistan; 4The University of Kansas Health System, Kansas City, KS
Introduction: Inflammatory bowel disease (IBD), which encompasses Crohn's disease (CD) and ulcerative colitis (UC), poses significant challenges due to its chronic nature and insufficient response to current therapies in many patients. Stem cell treatment has emerged as a promising therapeutic option for inducing remission and achieving mucosal healing. However, we have not thoroughly explored the efficacy and safety of stem cell-based treatments for long-term outcomes.
Methods:
Objective: To evaluate the efficacy and safety of stem cell therapy in treating IBD, focusing on clinical outcomes such as remission rates, mucosal healing, and adverse events.
Methods: We conducted a thorough review and meta-analysis of randomized controlled trials (RCTs) and observational studies evaluating the effectiveness of stem cell therapy for patients with inflammatory bowel disease (IBD). Detailed searches of databases such as PubMed, EMBASE, Cochrane Library, and ClinicalTrials.gov were conducted until December 2024. The primary outcomes were clinical remission, endoscopic mucosal healing, improved quality of life, and adverse events. A random-effects model was used to calculate pooled effect sizes and 95% confidence intervals (CIs). The I² statistic was employed to assess heterogeneity.
Results: Out of 720 studies screened, 25 met inclusion criteria, comprising 1,500 patients with IBD (Crohn's disease: n = 800; ulcerative colitis: n = 700). Stem cell therapy significantly improved clinical remission rates compared to standard care or placebo (pooled odds ratio [OR]: 2.5, 95% CI: 1.8-3.2, p < 0.05). Mucosal healing was observed in 60% of patients receiving stem cell therapy versus 35% in controls. Quality of life scores demonstrated significant improvement (standardized mean difference [SMD]: 0.8, p < 0.05). Adverse events were reported in 15% of patients, with no significant difference compared to controls (relative risk [RR]: 1.1, p = 0.3).
Discussion: Stem cell therapy has been shown to be very effective in achieving clinical remission and mucosal healing in IBD patients, with a favourable safety profile. Long-term follow-up studies are required to evaluate long-term benefits and rare adverse effects. This meta-analysis highlights the therapeutic potential of stem cell-based treatments as a new paradigm in IBD treatment.
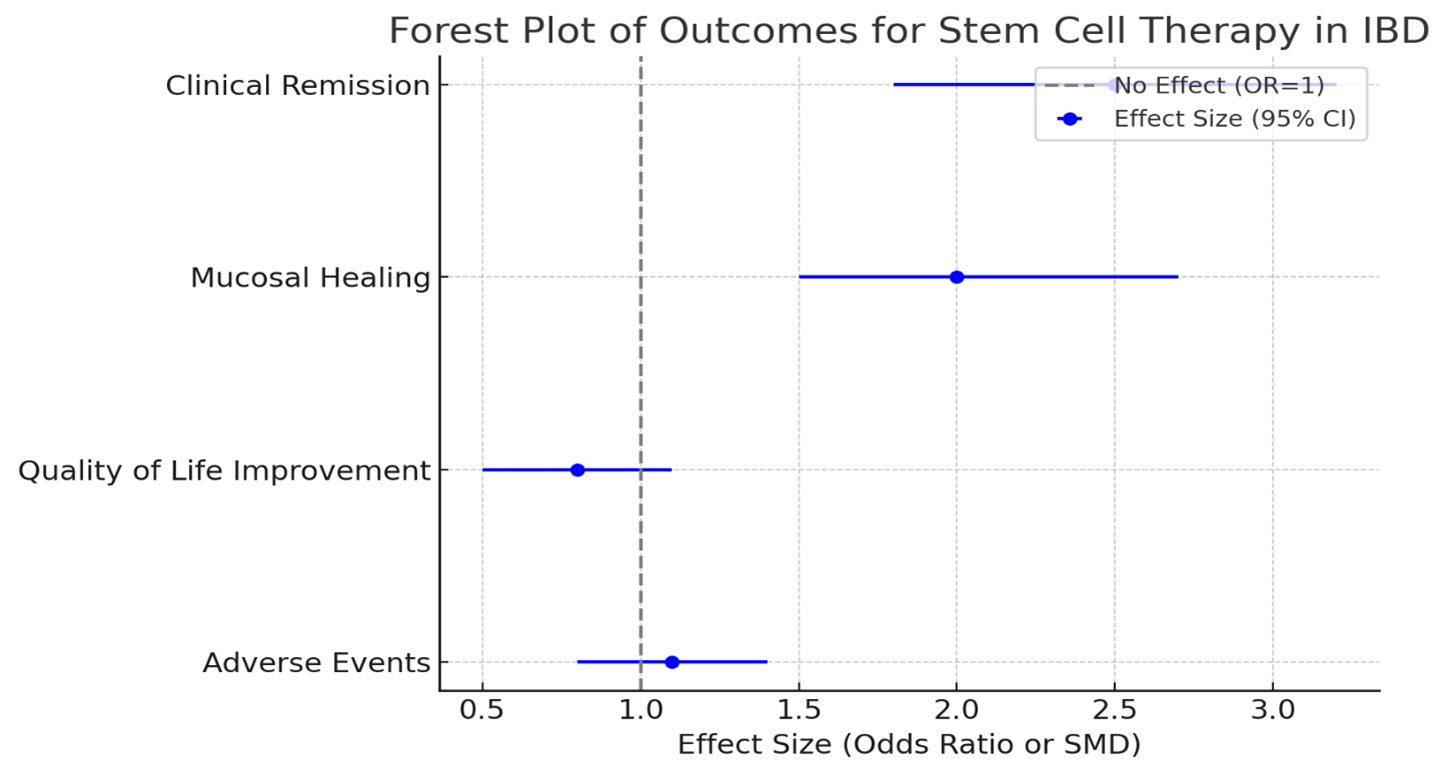
Figure: Figure 1: Efficacy and Safety of Stem Cell Therapy in IBD
Disclosures:
Angad Tiwari indicated no relevant financial relationships.
Sohini Samaddar indicated no relevant financial relationships.
Harendra Kumar indicated no relevant financial relationships.
Charlie Altfillisch indicated no relevant financial relationships.
Dushyant Dahiya indicated no relevant financial relationships.
Eric Molloy indicated no relevant financial relationships.
Angad Tiwari, MBBS1, Sohini Samaddar, MBBS2, Harendra Kumar, MBBS3, Charlie Altfillisch, MD2, Dushyant Dahiya, MD2, Eric Molloy, MD4. P3311 - Efficacy and Safety of Stem Cell Therapy in Treating Inflammatory Bowel Disease: A Comprehensive Meta-Analysis of Clinical Outcomes and Long-Term Effects, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Maharani Laxmi Bai Medical College, Ghaziabad, Uttar Pradesh, India; 2University of Kansas Medical Center, Kansas City, KS; 3Dow University of Health Sciences, Karachi, Sindh, Pakistan; 4The University of Kansas Health System, Kansas City, KS
Introduction: Inflammatory bowel disease (IBD), which encompasses Crohn's disease (CD) and ulcerative colitis (UC), poses significant challenges due to its chronic nature and insufficient response to current therapies in many patients. Stem cell treatment has emerged as a promising therapeutic option for inducing remission and achieving mucosal healing. However, we have not thoroughly explored the efficacy and safety of stem cell-based treatments for long-term outcomes.
Methods:
Objective: To evaluate the efficacy and safety of stem cell therapy in treating IBD, focusing on clinical outcomes such as remission rates, mucosal healing, and adverse events.
Methods: We conducted a thorough review and meta-analysis of randomized controlled trials (RCTs) and observational studies evaluating the effectiveness of stem cell therapy for patients with inflammatory bowel disease (IBD). Detailed searches of databases such as PubMed, EMBASE, Cochrane Library, and ClinicalTrials.gov were conducted until December 2024. The primary outcomes were clinical remission, endoscopic mucosal healing, improved quality of life, and adverse events. A random-effects model was used to calculate pooled effect sizes and 95% confidence intervals (CIs). The I² statistic was employed to assess heterogeneity.
Results: Out of 720 studies screened, 25 met inclusion criteria, comprising 1,500 patients with IBD (Crohn's disease: n = 800; ulcerative colitis: n = 700). Stem cell therapy significantly improved clinical remission rates compared to standard care or placebo (pooled odds ratio [OR]: 2.5, 95% CI: 1.8-3.2, p < 0.05). Mucosal healing was observed in 60% of patients receiving stem cell therapy versus 35% in controls. Quality of life scores demonstrated significant improvement (standardized mean difference [SMD]: 0.8, p < 0.05). Adverse events were reported in 15% of patients, with no significant difference compared to controls (relative risk [RR]: 1.1, p = 0.3).
Discussion: Stem cell therapy has been shown to be very effective in achieving clinical remission and mucosal healing in IBD patients, with a favourable safety profile. Long-term follow-up studies are required to evaluate long-term benefits and rare adverse effects. This meta-analysis highlights the therapeutic potential of stem cell-based treatments as a new paradigm in IBD treatment.

Figure: Figure 1: Efficacy and Safety of Stem Cell Therapy in IBD
Disclosures:
Angad Tiwari indicated no relevant financial relationships.
Sohini Samaddar indicated no relevant financial relationships.
Harendra Kumar indicated no relevant financial relationships.
Charlie Altfillisch indicated no relevant financial relationships.
Dushyant Dahiya indicated no relevant financial relationships.
Eric Molloy indicated no relevant financial relationships.
Angad Tiwari, MBBS1, Sohini Samaddar, MBBS2, Harendra Kumar, MBBS3, Charlie Altfillisch, MD2, Dushyant Dahiya, MD2, Eric Molloy, MD4. P3311 - Efficacy and Safety of Stem Cell Therapy in Treating Inflammatory Bowel Disease: A Comprehensive Meta-Analysis of Clinical Outcomes and Long-Term Effects, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
